Chapter 4 Inhibitory effect of nitric oxide (NO) synthase inhibitors on naloxone-precipitated withdrawal syndrome in morphine dependent mice
| Books - Modulators of Drug Dependence Phenomena |
Drug Abuse
Chapter 4 Inhibitory effect of nitric oxide (NO) synthase inhibitors on naloxone-precipitated withdrawal syndrome in morphinedependent mice
Abstract - The effect of intraperitoneally administered nitric oxide (NO) synthase inhibitors has been examined on the naloxone-precipitated withdrawal syndrome in morphine-dependent mice. L-NAME (30-200 mg/kg) and L-NOARG (7.5-50 mg/kg) induced a significant decrease of naloxone-precipitated withdrawal jumping and diarrhoea. However, L-NMMA (3.5-100 mg/kg), considered as a less potent NO synthase (NOS) inhibitor, did not significantly affect the withdrawal signs in mice. Although a specificity of NOS inhibitors is not fully established, these results indicate that inhibition of NO synthesis in the central nervous system and periphery may significantly affect the morphine withdrawal phenomena. Accordingly, this study suggests an involvement of NO in morphine withdrawal syndrome. Published in Neuroscience Letters 162: 97-100, 1993.
The cessation of chronic use of central depressant drugs is associated with excitatory withdrawal signs. This could suggest an involvement of excitatory neurotransmitters in drug dependence phenomena. Accordingly, a recent study indicates that kynurenic acid, a nonselective antagonist of excitatory amino acid (EAA) receptors, attenuated a naloxoneprecipitated withdrawal in rats (Rasmussen et al., 1991a). Similarly, other studies demonstrated that the non-competitive N-methyl-D-aspartate (NMDA) antagonist, MK-801, and the competitive NMDA antagonist, LY274614, suppressed the behavioral signs of withdrawal in morphine-dependent rats (Koyuncuoglu et al., 1992; Rasmussen et al., 1991b; Trujillo and Akil, 1991). We also observed that antagonists of various EAA receptors (NMDA-antagonist MK-801, non NMDA-antagonist DNQX and antagonist of glycine site of NMDA receptor 5,7-DCKA) attenuated the naloxone-precipitated withdrawal syndrome in morphine-dependent mice (Cappendijk et al., 1993).
Concerning nitric oxide (NO), it has been suggested that this compound is produced enzymatically in postsynaptic structures, in response to activation of central EAA receptors (Garthwaite et al., 1989; Knowles et al., 1989). Thus, there is a possibility that antiwithdrawal effect of antagonists of NMDA receptors might be due to the decrease of NO synthesis. In order to explore the proposed mechanism of action of antagonists of EAA transmission on drug withdrawal, we studied the effect of several NOS inhibitors Ng-nitro-L-arginine methyl ester hydrochloride (L-NAME), NG-nitro-L-arginine (L-NOARG) and N°-monomethyl-L-arginine acetate (L-NMMA) on naloxone-precipitated withdrawal in morphinedependent mice.
Materials and Methods
Morphine dependence
Chronic morphine dependence was induced in male Swiss mice (25-35 g) by implantation of pellet (25 mg morphine base/mouse, according to the method of Kosersky et al., 1974) under ether anaesthesia. The animals were housed individually in a room maintained on a 12
h light-dark cycle (lights on 08.00 h) with food and water ad libitum. The morphine withdrawal was precipitated by administration of naloxone (4 mg/kg, i.p.), 72 h after pellet implantation. The withdrawal severity was quantified by counting the frequency of jumping from a circular platform (30 cm high, 12 cm diameter). The presence of diarrhoea was checked.
Experimental protocol
The mice were divided into four groups, pretreated intraperitoneally with vehicle (distilled water, n=21), L-NAME (7.5-400 mg/kg, n=49), L-NOARG (3.5-100 mg/kg, n=36), or LNMMA (3.5-100 mg/kg, n=30) 30 min prior to naloxone. Based on our preliminary experiments and other studies (Moore et al., 1991; Morgan et al., 1992), we selected biologically active doses of these drugs, which do not alter the locomotor activity. Regarding the penetration of NOS inhibitors in CNS, it is known that these substances are lipophilic - particularly L-NAME, and therefore, may pass easily through various lipid membranes, including blood-brain barrier (Gardiner et al., 1990; Morgan et al., 1992; Rees et al., 1990).
A drug pretreated animal was placed on a platform and observed for the following 30 min. At the end of the 30-min period, naloxone was administered and withdrawal jumping behaviour was counted and withdrawal diarrhoea was checked, for the following 30 min. The pH of drug solutions was adjusted to 7-8 and all drug solutions were given in an equal volume (0.5 ml/injection, i.p.).
Statistics
Data are expressed as mean ± SEM, while the control group (animals pretreated with vehicle) was taken as 100%. The effects of drug and vehicle treatment were evaluated statistically using the non-parametric Kruskall-Wallis one-way analysis of variance, followed by the Mann-Whitney U-test, with a level of P<0.05 being considered significant (Glantz, 1989).
Results
Data in Fig. 1A show a significant and dose-related decrease of the withdrawal jumps following administration of L-NAME (30-200 mg/kg, i.p.). Similarly, L-NOARG (7.5-50 mg/kg, i.p.) induced a significant decrease of naloxone-precipitated withdrawal jumps (Fig. 1B)
However) further increase of concentrations of L-NAME (400 mg/kg, i.p.) and L-NOARG (100 mg/kg) failed to decrease a withdrawal jumping behaviour (Figs. IA and 1B). In contrast) L-NMMA (3.5-100 mg/kg) did not significantly decrease the naloxone-precipitated withdrawal jumping in mice (Fig. 1 C)
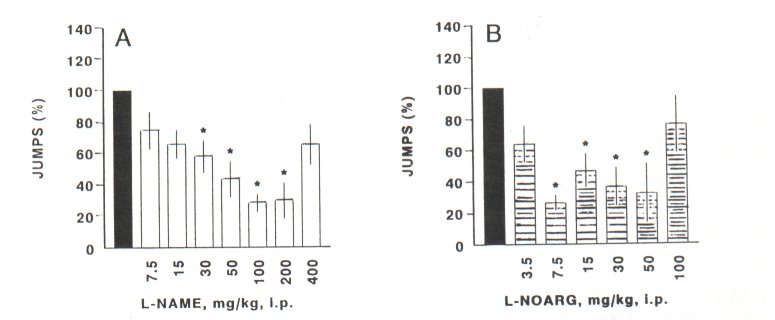
Fig. IA-C. Naloxone-precipitated withdrawal jumps in the morphine-dependent mice. Results are shown as mean jumps ± SEM during 30 min following naloxone (4 mg/kg, i.p.) administration. The numbers of jumps in the control group (•) pretreated with vehicle (n=21) was expressed as 100%. This group is the same in all three figures. Other animals were pretreated with L-NAME (O) Fig. IA) 7.5-400 mg/kg, i.p., 30 min prior naloxone; n=7) each dose group)) or with LNOARG (B) Fig. 1B) 3.5-100 mg/kg, i.p., 30 min prior naloxone; n=6) each dose group)) or with LNMMA (0) Fig. 1C) 3.5-100 mg/kg, i.p., 30 min prior naloxone; n=6) each dose group. Data in these figures were analyzed by the non-parametric Kruskill-Wallis ANOVA, one-way followed by the Mann-Whitney U-test.
* Significance at level of P<0.05
Withdrawal diarrhoea was present in 20 of the in total 21 tested control animals. LNAME and L-NOARG showed a dose-related inhibitory effect on the withdrawal diarrhoea. Sufficiently higher concentrations of L-NAME (50 mg/kg) and L-NOARG (100 mg/kg) completely abolished the morphine withdrawal diarrhoea in mice. However, L-NMMA in a dose range of 3.5-100 mg/kg had no significant effect on withdrawal diarrhoea (Table 1)
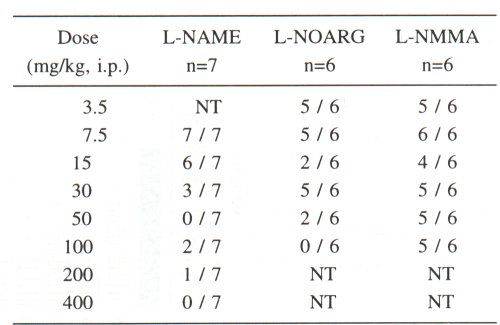
Table I. Naloxone-precipitated withdrawal diarrhoea in morphine-dependent mice, pretreated with NO synthase (NOS) inhibitors. The results are presented as a number of animals with withdrawal diarrhoea versus total number of observed mice in the corresponding dose group. In the control group of morphine-dependent mice pretreated with vehicle (n=21), the naloxone (4 mg/kg, i.p.)-precipitated withdrawal diarrhoea was observed in 20 animals. NT = not tested dose; n - number of animals in each dose group.
Discussion
The results of this study indicate that the NOS inhibitors, L-NAME and L-NOARG, significantly attenuated the naloxone-precipitated withdrawal jumping and diarrhoea in morphinedependent mice. However, L-NMMA in the dose range used in this study had no effect on withdrawal jumping behavior or diarrhoea in mice. This might probably be due to the fact that L-NMMA compared to the other NOS inhibitors is a significantly less potent drug (Garthwaite, 1991; Lambert et al., 1991).
In addition, we proposed that the anti-withdrawal effect of antagonists of EAA receptors is mediated by decreased activity of NO. This hypothesis was supported by results of this study, since the blockade of the NO synthesis attenuated the withdrawal syndrome in morphine-dependent mice. Administration of both single and continuous injection of L-NAME (100 mg/kg, s.c.; 12 mg/rat/day, respectively) to morphine-dependent rats also showed an attenuation of the naloxone-induced withdrawal syndrome (Adams et al., 1993). Accordingly, NO donor isosorbide dinitrate induced a quasi morphine-abstinence syndrome and aggravated the opioid withdrawal symptoms (Adams et al., 1993).
It is of interest to note that the higher dose of L-NAME (400 mg/kg, i.p.) and L-NOARG (100 mg/kg, i.p.) failed to attenuate the naloxone-precipitated withdrawal jumping. A similar phenomenon has been observed with high doses of EAA receptor antagonists (Koyuncuoglu et al., 1992; Cappendijk et al., 1993). The reason for this effect is not known, but an involvement of additional mechanism(s) activated by higher drug concentrations should be considered. For example, it is known that higher doses of EAA receptor antagonists exert a prominent excitatory/proconvulsant effect in animals (Jurson and Freed, 1990; Schoepp et al., 1990). This may aggravate a morphine withdrawal syndrome, since it is composed of mainly excitatory psychomotoric symptoms.
Pharmacological characteristics of NOS inhibitors are not yet fully clarified. The reason for decrease of withdrawal jumping in mice following NOS inhibitors is also not known. However, some reasonable possibilities could be suggested. NO has a substantial effect on presynaptic neurotransmitter release (Garthwaite, 1991). A derangement of this transmitter release, following administration of NOS inhibitors might significantly affect withdrawal syndrome. Noradrenaline could be one of the transmitters involved in the anti-withdrawal effect of NOS inhibitors. NO increases tyrosine hydroxylase and potentiates presynaptic catecholamine release (O'Sullivan and Burgoyne, 1990). A possible decrease of the central catecholamine release, following treatment with NOS inhibitors might have an attenuating effect on morphine withdrawal. Several studies provided evidence that the central noradrenergic system is hyperactive during opioid withdrawal syndrome (Tseng et al., 1975; Aghajanian, 1978). In addition, it is indicated that serotonin is also involved in the mechanisms which lead to compulsive jumping during naloxone-precipitated withdrawal (Cervo et al., 1981). However, the precise relationship between NO and serotonin release is not known.
The diarrhoea associated with morphine withdrawal is of both central and peripheral origin (Burks et al., 1988). A decrease in withdrawal diarrhoea following administration of NOS inhibitors L-NAME and L-NOARG might predominantly be due to decrease of periphe ral NO, since the muscle relaxation involved in the peristalsis is mediated by NO, synthesized in the neurons of the myenteric plexus (Snyder and Bredt, 1992). In addition, it has recently been demonstrated that alkyl esters of L-arginine (such as L-NAME) have anti-muscarinic properties (Buxton et al., 1993). Thus, it could be suggested that at least a part of the decrease in withdrawal diarrhoea might due to peripheral atropine-like activity of NOS inhibitors.
The NOS inhibitors used in this study are vasoconstrictors. However, there is no evidence that higher blood pressure attenuates opioid withdrawal. The blood pressure lowering agents - clonidine and NO donor isosorbide dinitrate - had an opposite effect on the opioid abstinence. Clonidine attenuated (Dionyssopoulos et al., 1992), while isosorbide dinitrate aggravated opioid withdrawal (Adams et al., 1993). Evidently, an alteration in blood pressure does not account for the anti-withdrawal effects of NOS inhibitors.
In conclusion, this study indicates an involvement of NO in the withdrawal syndromesince NOS inhibitors had a prominent attenuating effect on withdrawal jumping and diarrhoea. Furthermore, these results might support our hypothesis that the anti-withdrawal effect of EAA receptor-blocking drugs, observed by us and other authors, could be mediated by inhibition of NO synthesis. This could be further supported by the fact that the NO donor isosorbide dinitrate aggravates an opioid withdrawal (Adams et al., 1993). Although the mechanism of anti-withdrawal action of NOS inhibitors remains unknown, the complex changes in the central presynaptic neurotransmitter release, particularly decrease of noradrenaline, should be considered. In addition to the central NO, a decrease of peripheral NO synthesized in the myenteric plexus of gastro-intestinal tract and/or anti-muscarinic effect of NOS inhibitors might be of importance for attenuation of withdrawal diarrhoea.
References
Adams ML, Kalicki JM, Meyer ER and Cicero TJ, Inhibition of the morphine withdrawal syndrome by a nitric oxide synthase inhibitor, NG -nitro-L-arginine methyl ester, Life Sci 52: PL 245-249, 1993.
Aghajanian GK, Tolerance of locus coeruleus neurons to morphine and suppression of withdrawal response by clonidine, Nature 276: 186-188, 1978.
Burks TF, Fox DA, Hirning LD, Shook IE and Poreka F, Regulation of gastro-intestinal function by multiple opioid receptors, Life Sci 43: 2177-2181, 1988.
Buxton ILO, Cheek JD, Eckman D, Westwall DP, Sanders KM and Keef KD, N°-Nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists, Circ Res 72: 387-395,1993.
Cappendijk SLT, Vries R de, Dzoljic MR, Excitatory amino acid receptor antagonists and naloxoneprecipitated withdrawal syndrome in morphine-dependent mice, Eur Neuropsychopharmacol 3: 111-116, 1993.
Cervo L, Rochat C, Romandini S and Samanin R, Evidence of a preferential role of brain serotonin in the mechanisms leading to naloxone-precipitated compulsive jumping in morphine-dependent rats, Psychopharmacol 74: 271-274, 1981.
Dionyssopoulos T, Hope W and Coupar IM, Effect of adenosine analogues on the expression of opiate withdrawal in rats, Pharmacol Biochem Behav 42: 201-206, 1992.
Gardiner SM, Compton AM, Bennett T, Palmer MIR and Moncada S, Regional haemodynamic changes during oral ingestion of NG monomethyl-L-arginine or NG-nitro-L-arginine methyl ester in conscious Brattleboro rats, Br J Pharmacol 101: 10-12, 1990.
Garthwaite J, Southam E and Anderton M, A kainate receptor, linked to nitric oxide synthesis from arginine, J Neurochem 53: 1952-1954, 1989.
Garthwaite J, Glutamate, nitric oxide and cell-cell signalling in the nervous system, Trends Neurochem Sci 14: 60-67, 1991.
Glantz SA, Alternatives to analysis of variance and the t-test based on ranks, In: Primer of Biostatistics, B Kaufman-Barry and J White (eds.), McGraw-Hill, Singapore, pp. 291-317, 1989.
Jurson PA and Freed WJ, A slight anticonvulsant effect of CNQX and DNQX as measured by homocysteine- and quisqualate-induced seizures, Pharmacol Biochem Behav 36: 177-181, 1990.
Knowles RG, Palacios M, Palmer RMJ and Moncada S, Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase, Proc Natl Acad Sci USA 86: 5159-5162, 1989
Kosersky DS, Harris RA and Harris LS, Naloxone-precipitated jumping activity in mice following the acute administration of morphine, Eur J Pharmacol 26: 122-124, 1974.
Koyuncuoglu H, Dizdar Y, Aricioglu F and Sayin U, Effects of MK-801 on morphine physical dependence: Attenuation and intensification, Pharmacol Biochem Behav 43: 487-490, 1992.
Lambert LE, Whitten JP, Baron BM, Cheng HC, Doherty NS and McDonald IA, Nitric oxide synthesis in the CNS, endothelium and macrophages differs in its sensitivity to inhibition by arginine analogues, Life Sci 48: 69-75, 1991.
Moore PK, Oluyomi AO, Babbedge RC, Wallace P and Hart SL, L-NG-nitro arginine methyl ester exhibits antinociceptive activity in the mouse, Br J Pharmacol 102: 198-202, 1991.
Morgan CVJ, Babbedge RC, Gaffen Z, Wallace P, Hart SL and Moore PK, Synergistic antinociceptive effect of L-N °-nitro arginine methyl ester (L-NAME) and flurbiprofen in the mouse, Br J Pharmacol 106: 493-497, 1992.
O'Sullivan AJ and Burgoyne RD, Cyclic GMP regulates nicotine-induced secretion from cultured bovine adrenal chromaffin cells: effects of 8-bromo-cyclic GMP, atrial natriuretic peptide, and nitroprusside, J Neurochem 54: 1805-1808, 1990.
Rasmussen K, Krystal JH and Aghajanian GK, Excitatory amino acids and morphine withdrawal: differential effects of central and peripheral kynurenic acid administration, Psychopharmacology 105: 508-512, 1991a.
Rasmussen K, Fuller RW, Stockton ME, Perry KW, Swinford RM and Ornstein PL, NMDA receptor antagonists suppress behaviours but not norepinephrine turnover or locus coeruleus unit activity induced by opiate withdrawal, Eur J Pharmacol 197: 9-16, 1991b.
Rees DD, Schultz R, Hodson HF, Palmer RMJ and Moncada S, Identification of some novel inhibitors of the vascular nitric oxide synthase in vivo and in vitro, In: Nitric oxide from L-arginine: A Bioregulatory System, S Moncada and EA Higgs (eds.), Elsevier, Amsterdam, pp. 485-487, 1990.
Schoepp DD, Gamble AY, Salhoff CR, Johnson BG and Ornstein PL, Excitatory amino acid-induced convulsions in neonatal rats mediated by distinct receptor subtypes, Eur J Pharmacol 182: 421427, 1990.
Snyder SH and Bredt DS, Biological roles of nitric oxide, Sci Am 266: 28-35, 1992.
Trujillo KA and Akil H, Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801, Science 251: 85-87, 1991.
Tseng LF, Loh HH and Wei ET, Effects of clonidine on morphine withdrawal signs in rat,Eur J Pharmacol 30: 93-99, 1975
| < Prev | Next > |
|---|












