Chapter 7 The inhibitory effect of norharman on morphine withdrawal syndrome in rats: comparison with ibogaine
| Books - Modulators of Drug Dependence Phenomena |
Drug Abuse
Chapter 7 The inhibitory effect of norharman on morphine withdrawal syndrome in rats: comparison with ibogaine
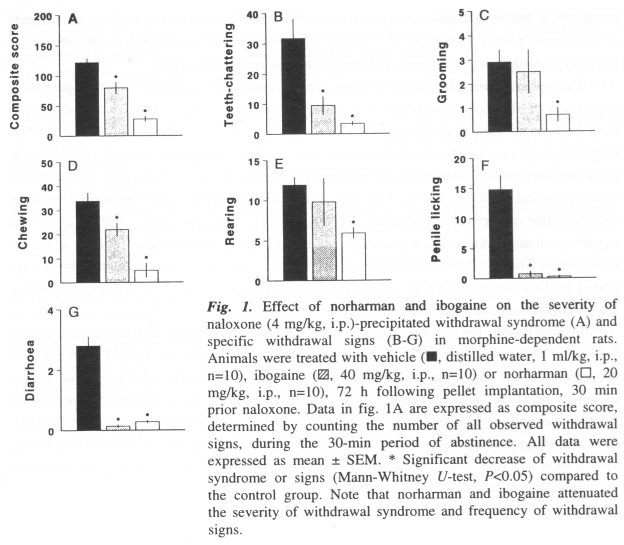
Abstract - Norharman (20 mg/kg, i.p.) and ibogaine (40 mg/kg, i.p.) significantly attenuated naloxone (4 mg/kg, i.p.)-precipitated withdrawal syndrome in morphine-dependent rats. Several withdrawal signs, such as teeth-chattering, chewing, penile licking and diarrhoea, were decreased by both norharman and ibogaine. In addition, norharman reduced also the withdrawal grooming and rearing. It is concluded that both norharman and ibogaine are inhibitors of withdrawal syndrome in morphine-dependent rats. Published in Behavioural Brain Research 65: 117-119, 1994.
Norharman (B-carboline) is an endogenous substance in brain and other tissues in rats and humans (Honecker and Coper, 1978; Greiner and Rommelspacher, 1984). Recently, elevated plasma levels of norharman were detected in chronic alcoholics (Rommelspacher et al., 1991) and heroin addicts (Stohler et al., 1993). These data favour the involvement of norharman in drug dependence processes. A substance structurally related to norharman is ibogaine. Both, norharman and ibogaine are indole derivatives with psychotogenic/ hallucinatory properties (Farnsworth, 1968; Airaksinen and Kari, 1981). It has been shown that ibogaine attenuates morphine withdrawal (Dzoljic et al., 1988; Glick et al., 1992) and interrupts drug dependence (Glick et al., 1991; Cappendijk and Dzoljic, 1993). These facts justify a further elucidation of the effects of these two substances in drug dependence phenomena. In order to make a comparison between these two chemically and behaviorally (psychotogenic/hallucinatory) similar substances, we studied the effects of both drugs, norharman and ibogaine on naloxone-precipitated withdrawal in morphine-dependent rats.
Materials and methods
Animals
Male Wistar rats (TNO Zeist), weighing 290-330 g were housed in groups and had a free access to food and water. The room was maintained on a 12-h light/dark cycle (lights on 08.00 h), with constant temperature (21' C) and humidity (55%).
Experimental protocol
Morphine dependence was induced by implantation of a morphine base pellet (75 mg/rat, s.c., n=30) on the back of rats the animal under ether anaesthesia (Cappendijk et al., 1993). The morphine-dependent animals were used only once. Morphine-dependent rats were divided into three groups, pretreated intraperitoneally with vehicle (distilled water, n=10), norharman (20 mg/kg, n=10) or ibogaine (40 mg/kg, n=10). The selected doses of norharman and ibogaine are biologically active, shown by previous studies (Morin, 1984; Cappendijk and Dzoljic, 1993).
Morphine withdrawal syndrome
The withdrawal syndrome in morphine-dependent animals was precipitated by naloxone (4 mg/kg, i.p.), given 30 min after vehicle, norharman or ibogaine. The naloxone treatment occurred 72 h following pellet implantation. The observer was "blind" to the treatment order and registered the withdrawal symptoms during 30 min following injection of naloxone. The withdrawal signs were scored according to the weighting factors described by Neal and Sparber (1986). In short, the signs observed during a mild withdrawal syndrome were assigned with 1 (diarrhoea, chewing, grooming, irritability on touch, rearing), whereas the sign rhinorrhoea, observed during severe withdrawal, was assigned a 3. All other withdrawal signs, teeth-chattering, wet-dog shakes, penile licking, ptosis and jumping were assigned by a weighting factor 2.
Drugs
Norharman (Sigma, England) and ibogaine (Sigma, England) were administered in volume of 2.2 ml/injection. Naloxone HCl (Sigma Chemical Co., St Louis, MO) was given in volume of 1 ml/kg. The pH of drug solutions and vehicles were adjusted to 7-8. All drugs were dissolved in distilled water.
Statistics
Data were evaluated by using the non-parametric Kruskall-Wallis one-way analysis of variance, followed by the Mann-Whitney U-test, with a level of P<0.05 being considered significant (Glantz, 1989).
Results
A decreased locomotion and exploratory behaviour was observed in norharman (20 mg/kg, i.p.)-treated naive (n=6) and morphine-dependent (n=10) animals. This effect lasted 5-20 min. In contrast, ibogaine (40 mg/kg, i.p.) induced within 4 min tremor and excitatory behaviour (jumping or violent locomotion on touch). The behavioral effects, induced by norharman or ibogaine disappeared within 30 min.
Norharman and ibogaine significantly attenuated the naloxone-precipitated withdrawal syndrome in rats (Fig. IA). Related to the specific symptoms, both norharman and ibogaine attenuated teeth-chattering, chewing, penile licking and diarrhoea (Fig. IB, D, F, G). Grooming and rearing response were reduced by norharman only (Fig. I C, E).
Discussion
This study is the first demonstration that norharman significantly attenuated a naloxone-precipitated withdrawal syndrome in rats. Ibogaine, in accordance with previous data (Dzoljic et al., 1988; Glick et al., 1992), also reduced naloxone-precipitated withdrawal syndrome (Fig. IA). However, the data indicate that norharman and ibogaine induced a similar (but not identical) decrease of opioid withdrawal symptoms.
Although the mechanism of action of norharman and ibogaine is not known, an involvement of the opioid system may be considered, since both drugs have an agonist action on opioid receptors. Norharman acts as a partial p-agonist (Airaksinen and Kari, 1981), while ibogaine is an agonist at K-receptors (Deecher et al., 1992). The binding activity of both drugs to central opioid receptors with possible displacement or preventing the binding of naloxone to opioid receptors may lead to an antiwithdrawal effect. In periphery, p and x-agonists can depress acetylcholine release from the cholinergic neurons of myenteric plexus (Burks et al., 1988). This effect may contribute to the decreased intensity of withdrawal diarrhoea, induced by norharman and ibogaine.
The other neurotransmitter system which could be involved in the decreased expression of the opioid withdrawal is the glutaminergic system. Glutamate antagonists may prevent morphine abstinence in mice and guinea pigs (Tanganelli et al., 1991; Cappendijk et al., 1993). Consistent with this finding, morphine is able to block the glutamate-mediated excitation in the monkey (Willcockson et al., 1986) and in the mouse (Aanonsen and Wilcox, 1987). The fact that both norharman and ibogaine also have morphine-like properties (Airaksinen and Kari, 1981; Deecher et al., 1992) favour the hypothesis that blockade of the glutamate-mediated transmission could contribute to the attenuation of the excitatory character of withdrawal syndrome. This idea has been supported by Dowson et al. (1975), showing that harmala alkaloids inhibit the transmission at the glutamate-mediated neurons.
In conclusion, the present experiments show that norharman and ibogaine attenuate the opioid withdrawal syndrome and favour an idea of an inhibitory role of both drugs in the expression of morphine abstinence. Although an involvement of the opioid- and or glutamate-neurotransmitter system could be considered as a main underlying mechanism for the attenuation of withdrawal syndrome, the precise mechanisms of action of norharman and ibogaine remain unclear. However, of particular importance would be a further clarification of the role of norharman as a physiological modulator of morphine withdrawal phenomena.
References
Aanonsen LM and Wilcox GL, Nociceptive action of excitatory amino acids in the mouse: Effects of spinally administered opioids, phencyclidine and sigma agonists, J Pharmacol Exp Ther 243: 9-19, 1987.
Airaksinen MM and Kari 1, B-Carbolines, psychoactive compounds in the mammalian body, Med Bio159: 190-211, 1981.
Burks TF, Fox DA, Himing LD, Shook JE and Porreca F, Regulation of gastrointestinal function by multiple opioid receptors, Life Sci 43: 2177-2181, 1988.
Cappendijk SLT, De Vries R and Dzoljic MR, Excitatory amino acid receptor antagonists and naloxone-precipitated withdrawal syndrome in morphine-dependent mice, Eur Neuropsycho pharmacol3: 111-116, 1993.
Cappendijk SLT and Dzoljic MR, Inhibitory effects of ibogaine on cocaine self-administration in rats, Ear J Pharmacol 241: 261-265, 1993.
Deecher DC, Teider M, Soderlund DM, Bommann WG, Kuehne ME and Glick SD, Mechanisms of action of ibogaine and harmaline congeners based on radioligand binding studies, Brain Res 571: 242-247, 1992.
Dowson RJ, Clements AN and May TE, The action of some harmala alkaloids on transmission at a glutamate-mediated synapse, Neuropharmacol 14: 235-240, 1975.
Dzoljic ED, Kaplan CD and Dzoljic MR, Effect of ibogaine on naloxone-precipitated withdrawal syndrome in chronic morphine dependent rats, Arch lnt Pharmacodyn 294: 64-70, 1988. Farnsworth NR, Hallucinogen plants, Science 162: 1086-1092, 1968.
Glantz SA, Alternatives to analysis of variance and the t-test based on ranks, In: Primer of Biostatistics, B Kaufman-Barry and J White (eds.), McGraw-Hill, Singapore, pp. 291-317, 1989.
Glick SD, Rossman K, Steindorf S, Maisonneuve IM and Carlson JN, Effects and aftereffects of ibogaine on morphine self-administration in rats, Eur J Pharmacol 195: 341-345, 1991. Glick SD, Rossman K, Rao NC, Maisonneuve IM and Carlson JN, Effects of ibogaine on acute signs of morphine withdrawal in rats: independence from tremor, Neuropharmacology 31: 497-500, 1992.
Greiner B and Rommelspacher H, Two metabolic pathways of tetrahydronorharman (tetrahydrobeta-carboline) in rats, Naunyn Schmiedeberg's Arch Pharmacol 325: 349-355, 1984. Honecker H and Coper H, Tetrahydronorharmane (THN) and 6-hydroxy-norharmane: physiological components in platelets and urine of man, Naunyn Schmiedeberg's Arch Pharmacol 302: R63, 1978.
Morin AM, f3-Carboline kindling of the benzodiazepine receptor, Brain Res 321: 151-154, 1984. Neal BS and Sparber SB, Mianserin attenuates naloxone-precipitated withdrawal signs in rats acutely or chronically dependent upon morphine, J Pharmacol Exp Ther 236: 157-165, 1986. Rommelspacher H, Schmidt LG, and May T, Plasma norharman (Q-carboline) levels are elevated in chronic alcoholics, Alc Clin Exp Res 15: 553-559, 1991.
Stohler R, Rommelspacher H, Ladewig D and Dammann G, Beta-carboline (harman/norharman) sind bei heroinabhangigen erhoht, Ther Umschau 50: 178-181, 1993.
Tanganelli S, Antonelli T, Morari M, Bianchi C and Beani L, Glutamate antagonists prevent morphine withdrawal in mice and guinea pigs, Neurosci Lett 122: 270-272, 1991.
Willcockson WS, Kim J, Shin HK, Chung JM and Willis WD, Actions of opioids on primate spinothalamic tract neurons, J Neurosci 6: 2509-2520, 1986.
| < Prev | Next > |
|---|












