Chapter 5 Comparative study of normotensive and hypertensive nitric oxide synthase inhibitors on morphine withdrawal syndrome in rats.
| Books - Modulators of Drug Dependence Phenomena |
Drug Abuse
Chapter 5Comparative study of normotensive and hypertensive nitric oxide synthase inhibitors on morphine withdrawal syndrome in rats.
Abstract - The effects of the normotensive, mainly centrally active nitric oxide synthase (NOS) inhibitor, 7-nitro indazole and the hypertensive compound N g-nitro-L-arginine, which blocks both the endothelial and central NOS, have been examined for their effects on naloxone-precipitated withdrawal syndrome in morphine-dependent rats. Both drugs attenuated the same withdrawal signs (teeth-chattering, penile licking, diarrhoea, chewing, wet-dog shakes, grooming), while other signs remained unaffected (rearing, jumping, ptosis, rhinorrhoea, irritability on touch). These findings indicate that mainly central (but not endothelial) nitric oxide is involved in the expression of some opioid withdrawal symptoms. Published in Neuroscience Letters 183: 67-70, 1995.
Activation of NMDA (N-methyl-D-aspartate) receptors stimulates synthesis of nitric oxide (NO, Garthwaite, 1991), while the blockade of these receptors attenuates naloxoneprecipitated withdrawal syndrome in morphine-dependent animals (Trujillo and Akil, 1991; Cappendijk et al., 1993a). This may implicate involvement of NO in the expression of morphine withdrawal syndrome. This idea received support from several experiments showing an attenuating effect of NO synthase (NOS) inhibitors on opioid withdrawal in mice (Kolesnikov et al., 1993; Cappendijk et al., 1993b; Majeed et al., 1994) and rats (Adams et al., 1993; Kimes et al., 1993). In addition, it has been suggested that NOS inhibitors block the development of morphine tolerance and dependence (Kolesnikov et al., 1993; Majeed et al., 1994). The NOS inhibitors used in all these studies inhibited the activity of both peripheral (endothelial) and central NOS. However, recently 7-nitro indazole (7-NI), which is a selective inhibitor for brain NOS, lacking effects on endothelial NOS and blood pressure (Moore et al., 1993a,b), has become available.
It is known that opioid withdrawal signs are affected by both central and peripheral factors (Neal and Sparber, 1986; Maldonado et al., 1992). In order to specify the role of central and peripheral NO in morphine-dependent rats, we compared the effects of 7-NI and N g-nitro-L-arginine (L-NOARG) on morphine withdrawal syndrome in rats. LNOARG inhibits both the central and the endothelial NOS and is a potent hypertensive agent. Hypertension is mainly due to the inhibition of endothelial synthesis and a corresponding reduction of vasodilatory NO (Iwata et al., 1992). This study shows that anti-withdrawal effect of NOS inhibitors is mainly due to a decrease in the activity of central NO.
Materials and methods
Animals
Male Wistar rats (TNO Zeist), weighing 290-330 g were housed in groups and had free access to food and water. The room was maintained on a 12-h light/dark cycle (lights on 08.00 h), with constant temperature (21° C) and humidity (55%).
Morphine dependence
Drug dependence in a rat was induced by s.c. implantation of 3 pellets, containing 25 mg morphine base/pellet, on the back of the animal under ether anaesthesia. The withdrawal syndrome was precipitated by administration of naloxone (4 mg/kg, i.p.) 72 h after pellet implantation (Cappendijk et al., 1993a). The observer was "blind" to the drug treatment procedure. The withdrawal symptoms were monitored for 30 min following injection of naloxone and scored according to the weighting factors described by Neal and Sparber (1986). In short, the signs observed during a mild withdrawal syndrome were assigned with I (chewing, diarrhoea, grooming, rearing, irritability on touch). Weighting factor 2 was given to the withdrawal signs, teeth-chattering, wet-dog shakes, penile licking, ptosis and jumping. The sign rhinorrhoea, observed during severe withdrawal was assigned a 3.
Experimental protocol
L-NOARG (7.5-100 mg/kg, i.p., n=35; Research Biochemical Incorporation, England) or vehicle (distilled water, i.p., n=12) were administered 30 min prior to naloxone. 7-NI (6.25-50 mg/kg, i.p., n=28; Sigma) or vehicle (arachis oil, i.p., n=9) were given 5 min prior to naloxone. These doses of L-NOARG and 7-NI have an inhibitory effect on NOS (Klatt et al., 1994; Moore et al., 1993b) and biological effect, for example on nociception (Babbedge et al., 1993; Moore et al., 1993a). The time interval between the administration of 7-NI or L-NOARG and naloxone was chosen to ensure a maximal inhibitory effect of these drugs on NOS (Klatt et al., 1994; Moore et al., 1993b) during observation of withdrawal signs.
Solutions of L-NOARG and 7-NI were given i.p. in a volume of 2.2 ml/injection. Naloxone was given i.p. in a volume of 1 mi/kg animal. The pH of drug solutions and vehicles were adjusted to 7-8. Each animal was used only once.
Statistics
The data were evaluated by using the non-parametric Kruskal-Wallis one-way analysis of variance, followed by the Mann-Whitney U-test, with a level of P<0.05 being considered significant
Results
The results show that no differences were observed with respect to the frequency of the withdrawal signs, between the controls treated with distilled water or arachis oil. Therefore, both controls were considered as a single group. The results illustrated in Fig. IA show that both 7-NI (12.5-50 mg/kg, i.p.) and L-NOARG (15-100 mg/kg, i.p.) attenuated significantly the severity of withdrawal syndrome (P=0.0001, P=0.0003, respectively) compared to the control group. Between 7-NI and L-NOARG treatment no differences were observed in the expression of the withdrawal syndrome (P=0.37). The withdrawal signs, teeth-chattering, penile licking, diarrhoea, chewing, wet-dog shakes and grooming (Fig. 1 B-G) were significantly attenuated by both 7-NI and L-NOARG. However, the effect of 7-NI was predominantly dose-related, while L-NOARG induced a U-shape dose-response curve in three out of six withdrawal signs. Other withdrawal signs, rearing, jumping, ptosis, rhinorrhoea and irritability on touch were not significantly altered by any of the NOS inhibitors used in this study (Fig. 1 H-L).
Discussion
This study demonstrated that both NOS inhibitors, 7-NI and L-NOARG induced a significant decrease of severity of the naloxone-precipitated withdrawal syndrome. The fact that some withdrawal symptoms were attenuated by both NOS inhibitors, implicates that these signs are predominantly affected by decreased concentrations of central NO. However, this does not exclude an additional involvement of peripheral NO.
The exact mechanism involved in the role of central NO in the withdrawal syndrome remains unknown. However, some possibilities related to the specific withdrawal signs may be considered:
Activation of the NO system stimulates release of several neurotransmitters, such as acetylcholine (ACh, Lonart et al., 1992; Prast and Philippu, 1992), noradrenaline (NA, Lonart et al., 1992), and dopamine (DA, Lonart et al., 1993). The occurrence of some withdrawal signs has been ascribed to the increased release of specific neurotransmitters. For example, wet-dog shakes were ascribed to activity of serotonin (5-HT) and NA. Accordingly, lesion of the locus coeruleus or administration of 5-HT blocking agents reduced wet-dog shakes (Bedard and Pycock, 1977; Maldonado and Koob, 1993); grooming was related to stimulation of dopamine D, receptors (Van Wimersma Greidanus et al., 1989), while the chewing response was elicited by stimulation of cholinergic system and reduced by anticholinergic drugs (Gunne et al., 1982). Thus, an inhibition of the NO system by NOS inhibitors and the corresponding decrease of neurotransmitter release may contribute to the attenuation of morphine withdrawal syndrome
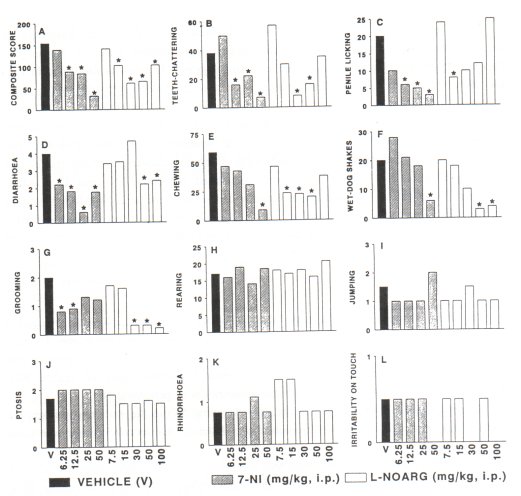
Fig. 1. Effect of the nitric oxide synthase (NOS) inhibitors 7-nitro indazole (7-NI) and N g-nitro-L-arginine (L-NOARG) on the severity of naloxone (4.0 mg/kg, i.pJ-precipitated withdrawal syndrome (A) and various withdrawal signs (B-L) in morphine-dependent rats. Animals were pretreated with 7-NI (®, 6.25-50 mg/kg, i.p., 5 min prior naloxone; n=7, each dose group) or with LNOARG (O, 7.5-100 mg/kg, i.p., 30 min prior naloxone; n=7, each dose group). The control animals, pretreated with vehicles (•, arachis oil, n=9, or distilled water, n=12) did not show differences in the severity of naloxone-precipitated withdrawal syndrome. Therefore, controls were considered as a single, vehicle-treated group (V, n=21). Data in fig. IA are expressed as composite score, determined by counting the number of all observed withdrawal signs, during the 30-min period of abstinence. The withdrawal signs were scored according to the method described by Neal and Sparber (1986) and all data were expressed as median values. * Significance at level of P < 0.05. Note that both NOS inhibitors attenuated the severity of the withdrawal syndrome and some of the withdrawal signs
In this study, NOS inhibitors also attenuated penile licking. Spinal cholinergic and NO-generating systems are known to be involved in the sensory regulation of the sympathetic and parasympathetic outflow to the penis (Krane et al., 1989; Zhuo et al., 1993). It seems that attenuation of the penile licking by 7-NI might be due to both a supraspinal insufficiency of the NO system (responsible for psychogenic erection) and decreased spinal release of ACh (responsible for the reflexogenic erection). In addition, the L-NOARG-induced attenuation of penile licking might be due to peripheral inhibition of NOS in non-adrenergic and non-cholinergic nerve terminals, which innervate the corpus cavernosum (Rajfer et al., 1992). The morphine withdrawal syndrome, therefore, could be used as an appropriate animal model to study the erectile mechanism.
Diarrhoea occurring during morphine withdrawal was usually considered as a peripheral effect (Maldonado et al., 1992), with some involvement of the CNS (Warhurst et al., 1984). The fact that both 7-NI and L-NOARG attenuated withdrawal diarrhoea favours the central component. However, an additional involvement of peripheral NO, following administration of L-NOARG could not be excluded, since the neurons of the myenteric plexus synthesize NO, which participates in the relaxation phase of peristalsis (Bredt et al., 1990).
Several other withdrawal symptoms (jumping, rearing, ptosis, rhinorrhoea and irritability on touch) were not altered by NOS inhibitors. This might indicate that NO is not involved in the expression of these signs.
An additional point of interest is the fact that attenuation of morphine withdrawal induced by L-NOARG is limited to a certain dose range. For some of the signs, the highest dose of L-NOARG failed to attenuate the naloxone-precipitated withdrawal symptoms. A similar phenomenon has been observed with high doses of NMDA receptor blockers (Koyuncuoglu et al., 1992; Cappendijk et al., 1993a) and NOS inhibitors (Cappendijk et al., 1993b) used for attenuation of withdrawal behaviour in rats and mice. The reason for this U-shaped dose-response curve is not known, but involvement of some additional mechanism(s) activated by higher drug concentrations should be considered. For example, a conversion of L-NOARG to L-arginine (Hecker et al., 1990) associated with accumulation of L-arginine and corresponding self-inhibition might be one of the explanations. The attenuating effect of 7-NI on withdrawal syndrome is predominantly dose-related.
In conclusion, this study indicates that central NO is involved in the expression of some (but not all) opioid withdrawal signs. Accordingly, an attenuation of the withdrawal syndrome, induced by NOS inhibitors is unrelated to an inhibition of endothelial NOS and increased blood pressure. We suggest that the anti-withdrawal effect of NOS inhibitors is due to a decrease of central NO levels and related decrease of neurotransmitters. This neurotransmitter derangement may affect the corresponding withdrawal signs in morphine dependent subject.
References
Adams ML, Kalicki JM, Meyer ER and Cicero TJ, Inhibition of the morphine withdrawal syndrome by a nitric oxide synthase inhibitor, N g-nitro-L-arginine methyl ester, Life Sci 52: PL 245-249,1993.
Babbedge RC, Hart SL and Moore PK, Anti -nociceptive activity of nitric oxide synthase inhibitors in the mouse: dissociation between the effect of L-NAME and L-NMMA, J Pharm Pharmacol 45: 77-79, 1993.
Bedard GG and Pycock CJ, 'Wet-dog' shake behaviour in the rat: a possible quantitative model of central 5-hydroxytryptamine activity, Neuropharmacol 16: 663-670, 1977.
Bredt DS, Hwang PM and Snyder SH, Localization of nitric oxide synthase indicating a neural role for nitric oxide, Nature 347: 768-770, 1990.
Cappendijk SLT, de Vries R and Dzoljic MR, Excitatory amino acid receptor antagonists and naloxone-precipitated withdrawal syndrome in morphine-dependent mice, Ear Neuropsy chopharmacol 3: 111-116, 1993a.
Cappendijk SLT, de Vries R and Dzoljic MR, Inhibitory effect of nitric oxide (NO) synthase inhibitors on naloxone-precipitated withdrawal syndrome in morphine-dependent mice, Neurosci Lett 162: 97-100, 1993b.
Garthwaite J, Glutamate, nitric oxide and cell-cell signalling in the nervous system, Trends Neurochem Sci 14: 60-67, 1991.
Gunne LM, Growdon J and Glaeser B, Oral dyskinesia in rats following brain lesions and neuroleptic drug administration, Psychopharmacol 77: 134-139, 1982.
Hecker M, Mitchell JA, Harris HJ, Katsura M, Thiemermann C and Vane JR, Endothelial cells metabolize N g-monomethyl-L-arginine to L-citrulline and subsequently to L-arginine, Biochem Biophys Res Comm 167: 1037-1043, 1990.
Iwata F, Job T, Kawai T and Itch M, Role of EDRF in splanchnic blood flow of normal and chronic portal hypertensive rats, Am J Physiol 263: G149-G154, 1992.
Kimes AS, Vaupel DB and London ED, Attenuation of some signs of opioid withdrawal by inhibitors of nitric oxide synthase, Psychopharmacol 112: 521-524, 1993.
Klatt P, Schmidt K, Brunner F and Mayer B, Inhibitors of brain nitric oxide synthase, J Biol Chem 269: 1674-1680, 1994.
Kolesnikov YA, Pick CG, Ciszewska G and Pasternak GW, Blockade of tolerance to morphine but not to k opioids by a nitric oxide synthase inhibitor, Proc Natl Acad Sci USA 90: 5162-5166, 1993.
Koyuncuoglu H, Dizdar Y, Aricioglu F and Sayin U, Effects of MK-801 on morphine physical dependence: Attenuation and intensification, Pharmacol Biochem Behav 43: 487-490, 1992.
Krane RJ, Goldstein I and Saenz de Tejada 1, Impotence, New Eng Med 321: 1648-1659, 1989. Lonart G, Wang J and Johnson KM, Nitric oxide induces neurotransmitter release from hippocampal slices, Eur J Pharmacol 220: 271-272, 1992.
Lonart G, Cassels KL and Johnson KM, Nitric oxide induces calcium-dependent [ 3H]dopamine release from striatal slices, J Neurosci Res 35: 192-198, 1993.
Majeed NH, Przewlocka B, Machelska H and Przewlocki R, Inhibition of nitric synthase attenuates the development of morphine tolerance and dependence in mice, Neuropharmacol 33: 189192, 1994.
Maldonado R, Stinus L, Gold LH and Koob GF, Role of different brain structures in the expression of the physical morphine withdrawal syndrome, J Pharmacol Exp Ther 261: 669-677, 1992.
Maldonado R and Koob GF, Destruction of the locus coeruleus decreases physical signs of opiatewithdrawal, Brain Res 605: 128-138, 1993.
Moore PK, Babbedge RC, Wallace P, Gaffen ZA and Hart SL, 7-Nitro indazole, an inhibitor of nitric oxide synthase, exhibits anti-nociceptive activity in the mouse without increasing blood pressure, Br J Pharmacol 108: 296-297, 1993a.
Moore PK, Wallace P, Gaffen ZA, Hart SL and Babbedge RC, Characterization of the novel nitric oxide synthase inhibitor 7-nitro indazole and related indazoles: antinociceptive and cardiovascular effects, Br J Pharmacol 110: 219-224, 1993b.
Neal BS and Sparber SB, Mianserin attenuates naloxone-precipitated withdrawal signs in rats acutely or chronically dependent upon morphine, J Pharmacol Exp Ther 236: 157-165, 1986. Prast H and Philippu A, Nitric oxide releases acetylcholine in the basal forebrain, Eur J Pharmacol 216: 139-140, 1992.
Rajfer J, Aronson WJ, Bush PA, Dorey FJ and Ignarro LJ, Nitric oxide as a mediator of relaxation of the corpus cavemosum in response to nonadrenergic, noncholinergic neurotransmission, New Eng Med 326: 90-94, 1992.
Trujillo KA and Akil H, Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801, Science 251: 85-87, 1991.
Van Wimersma Greidanus TB, Maigret C, Torn M, Ronner E, Van der Kracht S, Van der Wee NJA and Versteeg DHG, Dopamine D-1 and D-2 receptor agonists and antagonists and neuropeptide-induced excessive grooming, Eur J Pharmacol 173: 227-231, 1989.
Warhurst G, Smith GS, Higgs N, Tonge A and Tumberg LA, Influence of morphine tolerance and withdrawal on intestinal salt and water transport in the rat in vivo and in vitro, Gastroenterol 87: 1035-1041,1984.
Zhuo M, Meller ST and Gebhart GF, Endogenous nitric oxide is required for tonic cholinergic inhibition of spinal mechanical transmission, Pain 54: 71-78, 1993
| < Prev | Next > |
|---|












