Chapter 9 Inhibitory effects of ibogaine on cocaine self-administration in rats
| Books - Modulators of Drug Dependence Phenomena |
Drug Abuse
Chapter 9 Inhibitory effects of ibogaine on cocaine self-administration in rats
Abstract - In order to determine the potential anti-addictive properties of ibogaine, we used the cocaine self-administration model in rats. The results indicate that a single injection of ibogaine (40 mg/kg i.p.) produced a significant decrease of cocaine intake, which remained unaltered for more than 48 h. Since the half-life time of ibogaine is short, this might suggest the involvement of one or several active metabolites of ibogaine in cocaine intake. Repetitive administration of ibogaine on three consecutive days also induced a pronounced decrease of cocaine intake. However, a more prominent inhibitory effect on cocaine intake was observed in animals treated repeatedly with ibogaine (40 mg/kg i.p.), once each week for 3 consecutive weeks. These results indicate that ibogaine or its metabolite(s) is a long-lasting interruptor of cocaine dependence, which supports similar observations from uncontrolled clinical studies. Published in European Journal of Pharmacology 241: 261-265, 1993.
Ibogaine, an indole alkaloid found in the root bark of the African shrub Tabernanthe iboga, has been used in Gabon (West Central Africa) in low doses as a stimulant (combat fatigue, hunger and thirst) and in high doses for its hallucinogenic properties (religious rituals).
Recent animal studies and non-controlled observations in humans indicate that ibogaine may significantly affect drug dependence phenomena such as drug withdrawal and intake of addictive drugs. Accordingly, it has been demonstrated that ibogaine (i.c.v.) attenuated many (but not all) symptoms of naloxone-precipitated withdrawal in morphine-dependent rats (Dzoljic et al., 1988). A similar anti-withdrawal effect of ibogaine has been observed in morphine-dependent monkeys (Aceto et al., 1989) and rats (Glick et al., 1992).
Related to the intake of addictive drugs, it has been shown that ibogaine pretreatment decreases intravenous morphine self-administration in rats for several days (Glick et al., 1991). These results of animal experiments are in accordance with the long-lasting interruption of heroin abuse by ibogaine in humans (Lotsof, 1985). Ibogaine is also claimed to interrupt cocaine and amphetamine abuse and it was suggested that series of four treatments may be effective for several years (Lotsof, 1986). Other claims are that ibogaine attenuates alcohol and nicotine/tobacco dependency syndromes (Lotsof, 1989, 1991).
The aim of the present experiments was to determine whether an interrupting effect of ibogaine on cocaine intake could be demonstrated in cocaine-dependent animals. We examined the effects of single and repeated injections of ibogaine on the cocaine selfadministration model in rats.
Materials and methods
Animals
Male wistar rats. (TNO Zeist) were used, weighing 200-250 g at the start of the experiments. The animals were housed in groups with water and food ad libitum. Artificial light was supplied on a 12-h light/dark cycle.
Operation procedure
All animals were anaesthetized with sodium pentobarbital (60 mg/kg i.p.) and surgically implanted with a chronic i.v. jugular catheter (0.5 mm inside diameter, 1.0 mm outside diameter, polyethylene tubing). The catheter was passed subcutaneously to a small incision at the back of the neck. After the operation the animals were housed individually with food and water ad libitum. Two days before the start of the experiments (i.e. 5-6 days after operation), the animals were brought to the test room and were deprived of food in order to obtain a weight reduction of about 20%. Weight reduction was introduced in order to facilitate acquisition of self-administration (Takahashi et al., 1978). A reversed 12-h light/dark cycle (lights out 8.00 - 20.00 h) was maintained during the whole experiment.
Apparatus
The experiments were performed in operant conditioning chambers. Cocaine infusions (1.2 mg/kg), consisting of 0.25 ml fluid (pH 7.30-7.35) delivered in 20 s, occurred when the reinforcement lever was depressed. During the infusion, the stimulus light was turned off and pressing the same lever had no programmed consequences.
Test-procedure
Following 5-6 days of postoperative recovery, the rats were connected to an infusion pump (Braun Perfusor Secura MRD) by polyethylene tubing and a fluid swivel, which permitted unlimited movement of the animal during the session. Session length was 3 h each day (during the dark period of the cycle), 5 days per week with 2 days of no testing (during weekends, between each 5-day block of testing). The study of the effect of ibogaine began when the baseline rate of cocaine self-administration stabilized (< 10% variation between 3 consecutive sessions) after 12-16 days (sessions). These animals were randomly divided into vehicle and ibogaine-treated groups. The experiments lasted about 6 weeks (including the first 2 weeks used for stabilization of cocaine intake).
Experimental groups
Vehicle (1.0 ml/kg i.p.) or ibogaine was given 30 min prior to self-administration testing and the behaviour of animals was monitored for the subsequent 3 h.
Single administration of ibogaine (10-40 mg/kg i.p., n= 6-7 per dose). Our preliminary experiments showed that administration of 80 mg/kg ibogaine caused severe locomotor disturbances (ataxia, jumping when touched and tremor for about 60 min). Therefore, in further experiments, this dose was omitted and 40 mg/kg ibogaine was constantly used. This dose had less prominent and shorter lasting behavioral effects than the higher dose (see Results).
Repetitive administration of ibogaine (40 mglkg i.p.). In one group of animals (n=5) ibogaine was administered once on each of 3 consecutive days, while the other group (n=5) received ibogaine once at the beginning of each of 3 consecutive weeks.
Drugs
Cocaine hydrochloride (OPG, Utrecht, Netherlands) was dissolved in saline and the pH was adjusted to 7.30-7.35. Ibogaine hydrochloride (kindly donated by H. Lotsof, NDA, New York) was dissolved in distilled water.
Data analysis
Responses were summed over the 3-h test period and subjected to two-way analysis of variance (ANOVA) with repeated measurements on days. Individual comparisons of means were made with Student's t-test (between baseline and treated groups and between vehicle and ibogaine-treated groups) with significance at P<0.05 level.
Results
Single administration of ibogaine (10-40 mg/kg i.p. n= 6-7 per dose)
Behaviour. Administration of ibogaine in cocaine-dependent rats induced within 4 min stiffness of the hind legs, tremor, ataxia and hypersensitivity (jumping or , violent locomotion when touched). The severity of this behavioral syndrome was dose dependent and, in the case of the highest dose of ibogaine (40 mg/kg), the effect lasted for a maximum of 30 min. Thereafter, animals showed normal behaviour and were used for the self-administration procedure.
Cocaine intake. The baseline cocaine intake was 5.0 ± 0.5 mg/kg (Fig. 1). A single injection of 40 mg/kg ibogaine produced a significant depression of cocaine intake, while 10 and 20 mg/kg were ineffective (Fig. 1). The inhibitory effect of a single administration of ibogaine on cocaine intake became more prominent on the next day and remained below the control level for the 24 h following (48 h after drug administration, Fig. 1). Further studies were performed with the 40 mg/kg dose of ibogaine.
Repeated (three) administration of ibogaine (40 mglkg i.p.)
Ibogaine administered on each of 3 consecutive days. Compared to the baseline (5.3 ± 0.4), administration of vehicle (1.0 ml/kg i.p. n=5) on each of 3 consecutive days did not induce significant changes in cocaine intake (Fig. 2). However, a significant decrease of the cocaine intake (n=5) occurred on the second day of ibogaine treatment. After the third injection of ibogaine, the inhibitory effect on cocaine intake lasted for the next 24 h (Fig. 2). This effect on cocaine intake was not significantly different from that of a single injection of ibogaine, but was shorter (24h versus 48h)
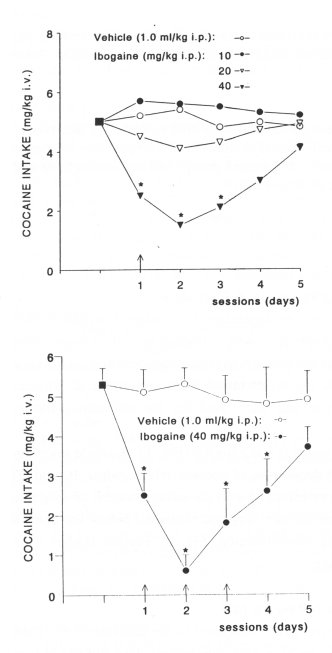 |
Fig. 1. Effect of a single dose of ibogaine (1040 mg/kg i.p. n= 6-7 per dose) on cocaine intake in rats. The baseline cocaine intake (0 5.0±0.5 mg/kg) was calculated as the average rate of three consecutive sessions (<10% variation) preceding treatment with vehicle (distilled water 1.0 ml/kg i.p. n=7) or ibogaine. Vehicle (T) or ibogaine (T) were administered 30 min before the session started. The data are expressed as means of cocaine intake per session. * Indicates a significant decrease of cocaine intake (ANOVA and t-test P<0.05) compared to baseline intake and vehicle-treated group. Note that a single injection of ibogaine (40 mg/kg) exerted a long-lasting (48 h) inhibition of cocaine intake.
Fig.2. Effect of repeated administration of ibogaine (40 mg/kg i.p. n=5, given once on each of three consecutive sessions) on cocaine intake in rats. The baseline cocaine intake (E 5.3±0.4 mg/kg) was calculated as the average rate of three consecutive sessions (<10% variation) preceding treatment with vehicle (distilled water 1.0 ml/kg i.p. n=5) or ibogaine. Vehicle (T) or ibogaine (T) was administered 30 min before the session started. The data are expressed as means ± SEM cocaine intake per session. Indicates a significant decrease of cocaine intake (ANOVA and t-test P<0.05) compared to baseline intake and vehicle-treated group. Note that each injection of ibogaine significantly decreased cocaine intake. |
Ibogaine administered at the beginning of each of .3 consecutive weeks. The baseline cocaine intake (4.9 ± 0.5) was not significantly affected by vehicle (1.0 ml/kg i.p. n=5) administered at the beginning of each of 3 consecutive weeks (Fig. 3). However, a significant decrease of cocaine intake was observed following each ibogaine injection. Compared to that after the first injection of ibogaine, the decrease of cocaine intake was more sustained after the second and third administration of ibogaine (Fig. 3).
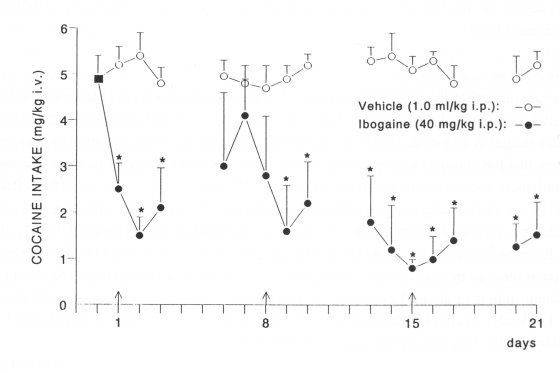
Fig. 3. Effect of repeated administration of ibogaine (40 mg/kg i.p. n=5, given once at the beginning of each of 3 consecutive weeks) on cocaine intake in rats. The baseline cocaine intake (m, 4.9±0.5 mg/kg) was calculated as the average rate of 3 consecutive sessions (<10% variation) preceding treatment with vehicle (distilled water 1.0 ml/kg i.p. n=5) or ibogaine. Vehicle (T) or ibogaine (T) was administered 30 min before the session started. The data are expressed as means ± SEM cocaine intake per session. The animals were not tested during weekends. * Indicates a significant decrease of cocaine intake (ANOVA and t-test P<0.05) compared to the baseline intake and the vehicle-treated group. Note a gradual and long-lasting decrease of cocaine intake following second and third injection of ibogaine.
Discussion
A single dose or repeated doses (on each of 3 consecutive days) of ibogaine (40 mg/kg i.p.) in rats induced a decrease of cocaine intake lasting 1-2 days. This effect could be potentiated and prolonged by three injections of ibogaine, given once each week (but not once each day). This was rather surprising, as the half-life time of ibogaine in rodents is about I h, and a day after administration, the ibogaine levels in the body were undetectable (Dhahir,1971, cited by Glick et al., 1991). This might indicate that the depression of cocaine intake could be ascribed to an active and long-lasting metabolite(s) of ibogaine or to irreversible interruption of the biological mechanism of cocaine dependence.
Related to the mechanism of anti-addictive properties of ibogaine several possibilities could be considered:
Disturbed locomotion
Ibogaine enhanced the amphetamine-induced increase of motor activity (Maisonneuve and Glick, 1992). Additional disturbances of motility, such as tremor and ataxia observed in this and other studies (Glick et al., 1991), might further affect the self-administration of cocaine. However, this possibility is unlikely, since in our experiments the ibogaine-induced locomotor disturbances such as ataxia and tremor lasted about 30 min, while the anti-addictive effect of single dose of this drug remained for at least 2 days. A long-lasting effect of ibogaine (several days) on morphine intake in rats was also observed in other studies (Glick et al., 1991). Ibogaine pretreatment of rats (40 mg/kg i.p. 19 h prior) had no effect on the increased locomotion induced by various doses of cocaine (5, 10 and 40 mg/kg), while the locomotion after administration of 20 mg/kg cocaine was potentiated for only 1 hour (Maisonneuve et al., 1992). Evidently, an effect of ibogaine on motor activity in rats is of marginal importance for understanding the long lasting anti-addictive properties of ibogaine.
Dopaminergic system
The rewarding effects of drugs of abuse have been associated with their ability to increase dopamine release, particularly in the nucleus accumbens (Di Chiara and Imperato, 1988). It is of importance to note that ibogaine reduced the cocaine-induced dopamine release in the nucleus accumbens (Broderick et al., 1992). Thus, an anti-addictive effect of ibogaine might be explained by its inhibitory effect on dopaminergic neurotransmission, which seems of importance for rewarding processes. However, the interaction between ibogaine and dopamine neurotransmission has not been shown conclusively, mainly due to controversial data. For example, a recent study indicated that ibogaine (40 mg/kg i.p.) potentiates the cocaine-induced increase in extracellular dopamine levels in striatum and nucleus accumbens (Maisonneuve et al., 1992). Thus, in contrast to the previous data, this might indicate a stimulatory effect of ibogaine on the reinforcing properties of cocaine.
Serotonergic system
Stimulation of the Serotonergic system by the 5-HT uptake inhibitor, fluoxetine, attenuates cocaine self-administration in animals (Richardson and Roberts, 1991). It has been shown that ibogaine inhibits the enzymic oxidation of 5-HT in the periphery (Barrass and Coult, 1972). However, it is not known whether such a relationship exists in the CNS. There seems to be no direct evidence that an ibogaine-induced derangement of 5-HT systems might affect the rewarding properties of cocaine. This possibility remains to be examined.
Central neuronal excitability
Ibogaine increases arousability (Schneider and Sigg, 1957), which might affect behaviour. The proconvulsant effect of ibogaine (40 mg/kg i.p.) lasting several hours that we observed in our EEG study (unpublished data), supports the idea that ibogaine significantly affects the responsiveness of central neurons. A proconvulsant state is probably incompatible with selfadministration behaviour. However, it is less clear why cocaine intake is decreased in the absence of a proconvulsant EEG pattern, more than 48 h after ibogaine administration.
In conclusion, these experiments indicate that ibogaine inhibits cocaine intake in rats. This effect could be potentiated by repeated injections of ibogaine, once each week. Although the mechanism of action of ibogaine remains to be established, the present results suggest the presence of an anti-addictive and long-lasting metabolite(s) of ibogaine or its irreversible/longlasting derangement of an addictive mechanism in cocaine-dependent animals.
References
Aceto MD, Bowman ER, Harris LS and May EL, Dependence studies on new compounds in the rhesus monkey, rat and mouse, NIDA Res Monogr 95: 578-630, 1989.
Barrass BC and Coult DB, Effects of some centrally acting drugs on caeruloplasmin, Prog Brain Res 36: 97-104, 1972.
Broderick PA, Phelan FT and Berger SP, Ibogaine alters cocaine-induced biogenic amine and psychostimulant dysfunction but not [ 3HIGBR-12935 binding to dopamine transporter protein, NIDA Res Monogr 119: 285, 1992.
Dhahir HI, A comparative study of the toxicity of ibogaine and serotonin (Doctoral dissertation, Indiana University), Univ Microfilms No 71-25: 341, 1971.
Di Chiara G and Imperato A, Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats, Proc Natl Acad Sci 85: 52745278,1988.
Dzoljic ED, Kaplan CD and Dzoljic MR, Effect of ibogaine on naloxone-precipitated withdrawal syndrome in chronic morphine dependent rats, Arch Int Pharmacodvn 294: 64-70, 1988.
Glick SD, Rossman K, Steindorf S, Maisonneuve IM and Carlson IN, Effects and aftereffects of ibogaine on morphine self-administration in rats, Eur J Pharnzacol 195: 341-345, 1991.
Glick SD, Rossman K, Rao NC, Maisonneuve IM and Carlson JN, Effect of ibogaine on acute signs of morphine withdrawal in rats: independence from tremor, Neuropharmacologv 32: 497-500, 1992.
Lotsof H, Rapid method for interrupting the narcotic addiction syndrome, Patent numnber 4,499,096, 1985.
Lotsof H, Rapid method for interrupting the cocaine and amphetamine abuse syndrome, Patent number 4,587,243, 1986.
Lotsof H, Rapid method for attenuating the alcohol dependency syndrome, Patent number 4,857,523, 1989.
Lotsof H, Rapid method for interrupting or attenuating the nicotine/tobacco dependency syndrome, Patent number 5, 026, 697, 1991.
Maisonneuve IM, Keller RW Jr and Glick SD, Interactions of ibogaine and D-amphetamine: in vivo microdialysis and motor behavior in rats, Brain Res 579: 87-92, 1992.
Maisonneuve IM and Glick SD, Interactions between ibogaine and cocaine in rats: in vivo microdialysis and motor behavior, Eur J Pharmacol 212: 263-266, 1992.
Richardson NR and Roberts DC, Fluoxetine pretreatment reduces breaking points on a progressive ratio schedule reinforced by intravenous cocaine self-administration in the rat, Life Sci 49: 833840, 1991.
Schneider JA and Sigg EB, Neuropharmacological studies on ibogaine, an indole alkaloid with centralstimulant properties, Ann NY Acad Sci 66: 765-776, 1957.
Takahashi RN, Singer G and Oei TP, Schedule induced self-injection of D-amphetamine by naive animals, Pharmacol Biochem Behav 9: 857-861, 1978.
| < Prev | Next > |
|---|












