Chapter 6 "Withdrawal substance" in cerebrospinal fluid of morphine-abstinent rats
| Books - Modulators of Drug Dependence Phenomena |
Drug Abuse
Chapter 6 "Withdrawal substance" in cerebrospinal fluid of morphine-abstinent rats
Abstract - The behavioral, electrophysiological (visual evoked potentials, VEP) and in vitroeffects of cerebrospinal fluid (CSF) taken from the donor rat have been investigated in the recipient rat and guinea-pig isolated ileum. The CSF of spontaneous morphine-abstinent donor rat precipitated in morphine-dependent recipient rat an opioid withdrawal syndrome, which was characterized by a decrease in the VEP peak latency N3 and amplitudes N2P3 and P3-N3. The CSF-induced withdrawal syndrome was behaviorally less severe and electrophysiologically less prominent, but qualitatively - identical to the naloxone-induced abstinence. However, in contrast to naloxone, the CSF from spontaneous morphine-abstinent rat did not contract the morphinedependent isolated guinea-pig ileum. Chromatographic analysis of CSF samples from naive, morphine-dependent or morphine-abstinent rats reveal distinct fractions, containing an active component present only in CSF of morphine-abstinent rats. The estimated relative molecular mass of this active component was around 50 kDa and the short retention time on the reversedphase column suggests the high hydrophobicity. The results indicate that spontaneous morphine-abstinent donors synthesize and release certain quantity of putative "withdrawal substance" in the CSF, which is without naloxone-like properties. This further suggests, that the CSF- and naloxone-precipitated withdrawal in the morphine-dependent recipients are mediated by activation of different neuronal mechanisms. Part of these data are published in Regulatory Peptides 1: 5227-S228, 1994.
It was shown that cerebrospinal fluid (CSF) from morphine-abstinent donor rats, precipitates an opiate withdrawal syndrome in morphine-dependent recipient rats (Malin et al., 1987). These authors found an increased level of octapeptide F-8-F-NH2 (Phe-Leu-PheGln-Pro-Gln-Arg-Phe-NH Z)-like immunoreactivity in CSF of morphine-dependent rats (Malin et al., 1990a). The octapeptide F-8-F-NH2 precipitates withdrawal syndrome in morphine-dependent rats, but not in the naive ones (Malin et al., 1990b). However, the role of this peptide in the CSF during morphine dependence and withdrawal remains unclear.
Opioid withdrawal is an excitatory syndrome, characterized by psychomotor activation, in both animals and men. The behavioral activation implicates an increase of neuronal excitability during morphine abstinence. This has been supported by finding of increased cell firing during opioid withdrawal (Aghajanian, 1978). Accordingly, a similar behaviour and increase of neuronal excitability should be expected following administration of CSF from morphine-abstinent donor rat into morphine-dependent recipient rat.
In order to test this hypothesis, we examined the electrophysiological effects of CSF withdrawn from abstinent rats, by monitoring the peak latencies and amplitudes of visual evoked potentials (VEP) during CSF-precipitated withdrawal syndrome in rats. The effect of CSF from spontaneous morphine-abstinent rats was also studied in naive rats, morphine-dependent rats and morphine-dependent isolated guinea-pig ileum. In addition, we analyzed CSF samples from morphine-abstinent, morphine-dependent and naive rats by high-performance liquid chromatography (reversed-phase and gel-filtration techniques).
Materials and methods Animals
Male Wistar rats (TNO, Zeist), weighing 200-300 g were housed in groups and had a free access to food and water. The room was maintained on 12 h light-dark cycle (lights on 8.00 h), with constant temperature (21° C) and humidity (55%).
Procedures Surgical procedure
All animals were anaesthetized with pentobarbital (60 mg/kg, i.p.). The donor rats were implanted with a chronic cannula, placed into the cisterna magna in order to withdraw CSF from conscious animals (Bouman and Van Wimersma Greidanus, 1979). In recipient rats, receiving CSF from donor rats, a cannula was placed into the lateral ventricle (Paakkari, 1980). Rats, involved in the VEP experiments were all recipient rats and, in addition to the lateral ventricle cannula, they were implanted with stainless steel screw electrodes over the right and left visual cortex (7 mm posterior to the bregma and 3 mm lateral to the midline). A reference electrode was placed in the frontal sinus. The electrodes were soldered to a miniature socket and attached to the skull with dental acrylate. In the recovery period (7 days), all operated animals were housed individually with food and water ad libitum. At the end of the experiments, the placement of lateral ventricle cannula was confirmed by injection of methylene blue.
Morphine dependence and abstinence
Morphine dependence was induced by treating animals with morphine for 8 days (twice daily, 9h and 17h). A starting dose of morphine was 10 mg/kg/injection, which increased daily to 20, 20, 40, reaching a final dose of 80 mg/kg/injection on the 5t h day. Rats treated with distilled water (1.0 mi/kg) for 8 days (twice daily, 9h and 17h), formed a group of naive animals. Morphine-dependent rats were considered as spontaneous morphine-abstinent, 6 h following the last morphine injection.
The control experiments were performed in naive and morphine-dependent rats on the 7th day of drug treatment. The behavioral signs similar to withdrawal symptoms occurred sporadically following administration of vehicle or artificial CSF. On the next day (8t h day of drug treatment), the animals were treated with naloxone or CSF from naive, morphinedependent or morphine-abstinent donor rats. The behaviour of the animals on the 7t h or 8t h day of drug treatment was monitored for 30 min following drug administration and scored by a person "blinded" to the experimental procedure.
The withdrawal signs were scored according to the weighting factors described by Neal and Sparber (1986). In short, the signs observed during a mild withdrawal syndrome were assigned with 1 (chewing, diarrhoea, grooming, rearing, irritability on touch). The weighting factor 2 was given to the withdrawal signs, teeth-chattering, wet-dog shakes, penile licking, ptosis and jumping. The sign rhinorrhoea, observed during severe withdrawal was assigned a 3.
Visual Evoked Potentials (VEP)
A postoperative recovery period of 7 days was followed by habituation of the recipient to the recording procedure. The method used for recording of VEP is described in the previous study of Dzoljic et al., (1994). Briefly, the animal was placed in a test chamber and after connecting the electrodes, a flash stimulus was induced at 1 per 7 s for 10 min. The habituation period lasted 3 days. This approach was selected, since it has been shown that under these conditions VEP discharges stabilize after several days (Bigler, 1975). The flash light was generated by a Grass S44 stimulator in a frequency of 0.14 Hz. Brain responses were amplified with a Grass model 79 B, connected to an analog-digital convertor (Lab Master, Scientific solutions Inc., Ohio, USA), triggered by the Grass S44 stimulator after every flash light. A computer connected to the analog-digital convertor performed the averaging of 25 VEP over a 800 ms epoch after every flash light and printed the results. Stimulation was performed only in animals with open eyes. The VEP parameters (peak latency and amplitude) were recorded in a total of six sessions, namely 5, 15 and 30 min before drug administration (self-control) and in the same time intervals after drug administration.
In vitro experiments
Male guinea-pigs (n=5, 600-900 g) were killed by a blow on the head. A 40 cm long segment of the small intestine was rapidly removed and placed in Krebs solution (room temperature). The terminal section of the guinea-pig ileum was used after discarding the portion of 10 cm closest to the ileo-caecal junction (Munro, 1953). The ileum was cut in eight 3-cm long segments. These segments were gently and thoroughly washed free of faecal matter by flushing Krebs solution through the lumen. Each streap of ileum was set up in a 8 ml organ bath containing Krebs solution and bubbled with 95% O z and 5% CO 2. Every 15 min the bath was perfused with fresh warm Krebs solution. The temperature and pH of the Krebs solution were maintained at 37°C and 7.4, respectively. The ileum was fixed at a resting tension of 1 g and allowed to equilibrate for 30 min. No drug was added in this time period. The spontaneous activity of the ileum was recorded isometrically. In order to induce morphine dependence, the ileum was exposed to morphine (1 uM) for 2 h (Cruz et al., 1991). The pieces of ileum not treated with morphine were considered as naive ileum. Exposure of ileum to CSF for 5 min was followed (after washing) by naloxone (0.1 uM) Naloxone remained in the bath also for 5 min. The CSF was made artificially or withdrawn from the donor rats (naive, morphine-dependent or morphine-abstinent) on the 8th day of drug treatment.
The contraction of the ileum was defined as the peak tension observed within 1 min after drug administration. In order to check the contractility of smooth muscle, each ileum was exposed to methacholine (0.1 uM) at the end of experiment. Only experiments with morphine-dependent ileum responding to methacholine and naloxone were taken as valid.
High-Performance Liquid Chromatography (HPLC)
Pooled samples of CSF (approximately 240-300 ul total volume) taken from naive, morphine-dependent or morphine-abstinent rats were analyzed using the SMART micropurification system (Pharmacia Biotech., Uppsala, Sweden). The system was operated as described in previous reports (Nyberg et al., 1991; Renlund et al., 1993). Briefly, a reversed-phase column pRPC C2/C18 (2.1 x 10 mm) and a gel-filtration column Superdex 75 (3.2 x 300 mm) were used in this study. The CSF samples were filtered through a nonsterile 45 um filter (Ultrafree-MC, Millipore, Bedford, MA, USA) and injected into the system. The reversed-phase column was eluted with a 30 min linear gradient from 0-60% acetonitrile, supplemented with 0.1% trifluoroacetic acid. The flowrate was maintained at 50 pl/min and one-min fractions were collected. The size separations (100 pl sample injected) were conducted using 20 mM Tris-HCI buffer of pH 7.4 as the eluent. The collected material was stored at -80° C until assayed.
Drugs
Morphine hydrochloride (OPG, Utrecht) and naloxone hydrochloride (Research Biochemical Incorporation, England) were dissolved in distilled water. The composition of solutions (expressed in mM) was as follows: Krebs buffer - NaCl 118; KCl 4.7; CaC1 2 2.5; NaHCO3 25; KH 2PO 4 1.2; MgSO 4 1.2; glucose 5.55; Artificial cerebrospinal fluid - NaCl 138; KCl 3.3; CaC 2 2.2; MgC1 2 1.15; NaHCO3 2.1; NaH 2PO 4 0.6; urea 2.16; glucose 3.38. The volume of CSF administered i.c.v. was 80 ± 5 ul per recipient rat.
Statistical analysis
The data in relation to withdrawal behaviour and electrophysiological study were statistically evaluated by using the non-parametric Kruskal-Wallis one-way analysis of variance, followed by Mann-Whitney U-test, with a level of P<0.05 being considered significant.
Results
Behaviour
Naive rats - recipients of CSF (Fig. IA)
Administration of artificial CSF (80 ± 5 pl, i.c.v.) into naive animals (n=15) treated with vehicle for 7 days, did not alter the normal behaviour, characterized with occasional appearance of grooming, digging, scratching and rearing. On the following day (8' h day of the vehicle treatment) these animals, randomly divided into three groups, received the CSF
(80 ± 5 ul, i.c.v.) taken from the naive rats (n=5), morphine-dependent (n=4) or spontaneous morphine-abstinent rats (n=6). The behaviour of animals in all three groups receiving various samples of CSF remained unaltered.
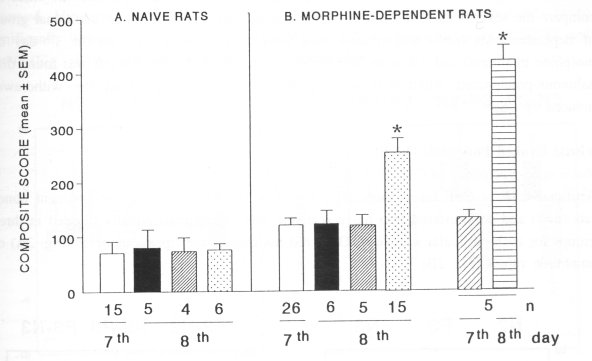
Fig. 1. Effect of CSF (80 ± 5 pl, i.c.v.) on the behaviour of naive (A) and morphine-dependent (B) rats. Both groups of animals were injected with artificial cerebrospinal fluid (0, i.c.v.) on the 7m day of drug (vehicle/morphine) treatment. The following day (8t h day), the animals were treated with cerebrospinal fluid (CSF) taken from the naive (0), morphine-dependent (®) or spontaneous morphine-abstinent rats (0). An additional group of morphine-dependent rats was treated with vehicle (0, 1.0 ml/kg, i.p., on the 7'h day of morphine treatment) and naloxone (0, 4.0 mg/kg, i.p. on the following day). Numbers beneath the bars represent the number of animals (n) and the day of treatment with vehicle or morphine. Data are expressed as composite score (mean ± SEM) by counting the number of all behavioral signs occurring in the naive and morphine-abstinent rats. The behaviour was monitored for 30 min. The behavioral signs were scored according to the method of Neal and Sparber (1986). * indicates significant differences (P<0.05) between the control and drug treatments. Note that CSF from the spontaneous morphine-abstinent rats (0) induced in morphine-dependent recipient rats (but not in the naive ones) a withdrawal syndrome, which is significantly less severe than the naloxone-induced withdrawal (0).
Morphine-dependent rats - recipients of CSF or treated with naloxone (Fig. IB) Administration of artificial CSF into dependent animals (n=26, on the 7th day of morphine treatment) did not change the behaviour. On the following day (8 th day of morphine treatment) these animals, randomly divided into three groups, received CSF obtained from the naive (n=6), morphine-dependent (n=5) or spontaneous morphine-abstinent (n=15) donor rats. No behavioral changes were observed in the morphine-dependent rats, receiving CSF from the naive or morphine-dependent donors. However, morphinedependent recipients treated with CSF taken from spontaneous morphine-abstinent rats exhibited a significant increase in the expression of withdrawal syndrome. In order to compare the severity of CSF- and naloxone-precipitated withdrawals, an additional group of dependent rats (n=5) was treated with vehicle (1.0 ml/kg, i.p., on the 7th day of morphine treatment) and naloxone (4.0 mg/kg, i.p. on the following day). It was found that naloxone-precipitated withdrawal was significantly more severe than the withdrawal induced by CSF.
Visual Evoked Potentials (VEP) Naive rats - recipients of CSF (Fig. 2) Artificial CSF or CSF taken from naive (n=5) or spontaneous morphine-abstinent donor rats (n=5) and administered into naive recipient rats (15 animals equally divided in three groups for each particular sample of CSF) did not change their peak latencies (Fig. 2A) or amplitude values (Fig. 2B).
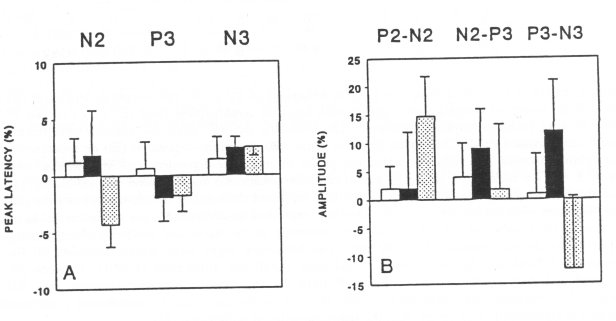
Fig. 2. Effect of CSF (80 ± 5 VI, i.c.v.) on the peak latencies (A) and amplitude values (B) of VEP in naive recipient rats. The artificial CSF (O) or CSF withdrawn from the naive (•, n=5) or spontaneous morphine-abstinent (0, n=5) donor rats were administered into three groups of naive recipient rats (5 animals for each different sample of CSF). The 0%-line, taken as self-control indicates the average of peak latencies and amplitude values, measured in three sessions (5, 15 and 30 min) before administration of CSF. Data are expressed as % ± SEM of altered peak latencies and amplitude values compared to self-control. Note that none of the CSF samples altered VEP parameters (latency and amplitude) in native recipients rats.Morphine-dependent rats - recipients of CSF (Fig. 3)
Artificial CSF or CSF taken from the naive donors (n=5) were administered to morphinedependent rats (10 animals equally divided into two groups for each particular sample of CSF). No significant changes in the peak latencies (Fig. 3A) or amplitudes (Fig. 313) were observed. However, CSF taken from spontaneous morphine-abstinent donor rats (n=6) significantly decreased the peak latency N3 (Fig. 3A), and amplitude values of N2-P3 and P3-N3 (Fig. 313) in morphine-dependent recipients (n=6).
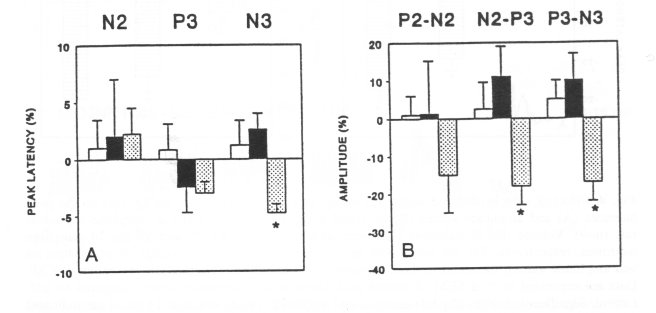
Fig. 3. Effect of CSF (80 + 5 pl, i.c.v.) on peak latencies (A) and amplitude values (B) of VEP in morphine-dependent recipient rats. CSF made artificially (0) or CSF withdrawn from the naive (I•, n=5) or spontaneous morphine-abstinent (O, n=6) donor rats were administered into morphinedependent recipient rats (5-6 animals for each different sample of CSF). The 0%-line, taken as selfcontrol indicates the average of peak latencies and amplitude values, measured in three sessions (5, 15 and 30 min) before administration of CSF. Data are expressed as % ± SEM of altered peak latencies and amplitude values compared to self-control. Significant changes of peak latencies and amplitude values versus self-control are indicated by * (P<0.05). Note that CSF from spontaneous morphine-abstinent donors (0) induced a significant decrease of peak latency N3 and amplitude values (N2-P3, P3-N3) of VEP in morphine-dependent rats.
Morphine-dependent rats - treated with vehicle and naloxone (Fig. 4)
Morphine-dependent animals (n=9) treated with vehicle, did not show changes in peak latencies (Fig. 4A) or amplitude values (Fig. 4B). However, the administration of naloxone to the same animals (n=9) on the next day (8th day of the morphine treatment) induced a significant decrease of the peak latencies P3 and N3 (Fig. 4A) and the amplitude values N2-P3 and P3-N3 (Fig. 4B).
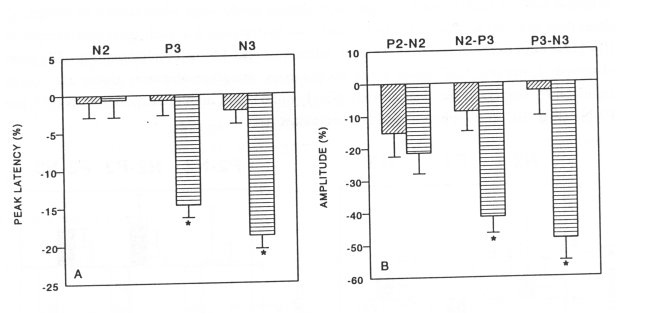
Fig. 4. Effect of vehicle (distilled water, 1.0 ml/kg, i.p.) and naloxone (4.0 mg/kg, i.p.) on the peak latencies (A) and amplitude values (B) of visual evoked potentials (VEP) in morphine-dependent rats (n=9). Vehicle (E0) or naloxone (0) were administered on the 7`" and 8t h day of morphine treatment, respectively. The 0%-line, taken as self-control indicates the average of peak latencies and amplitude values, measured in three sessions (5, 15 and 30 min) before administration of CSF. Data are expressed as % ± SEM of altered peak latencies and amplitude values compared to selfcontrol. Significant changes of peak latencies and amplitude values versus self-control are indicated by * (P<0.05). Note that naloxone induced a significant decrease of peak latencies (P3 and N3) and amplitude values (N2-P3 and P3-N3) of VEP in morphine-dependent rats.
In vitro experiments Guinea-pig ileum (Fig. 5) Administration of naloxone (0.1 uM) to the bath with morphine-dependent ileum (n=5) was followed by clear contractions (Fig. 5B), while the tonus of the naive ileum (n=5) remained unaltered (Fig. 5A). However, the artificial CSF or CSF taken from the naive (n=3), morphine-dependent (n=3) or spontaneous morphine-abstinent rats (n=4) did not affect the basal tonus of isolated naive or morphine-dependent guinea-pig ileum (Fig. 5A and 5B). Methacholine (0.1 uM) induced a contraction of both, naive and morphine dependent ileum (Fig. 5A and 5B).
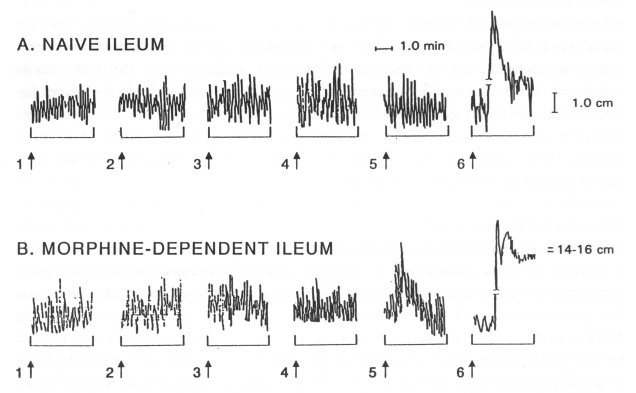
Fig. 5. Effect of CSF (80 ± 5 pl, 1>, 2T, 3T, 4T), naloxone (0.1 uM, 5T) and methacholine (0.1 uM, 6T) on naive and morphine-dependent guinea-pig ileum in vitro. The CSF added into the bath was made artificially (1>) or withdrawn from the naive (2T), morphine-dependent (3T) or spontaneous morphine-abstinent (4T) donor rats. Note that no any sample of CSF (1>, 2T, 3T, 4T) had an effect on muscle tonus, while naloxone (5T) induced a contraction only in the morphinedependent ileum. Methacholine (6T) induced a contraction in both, naive and morphine-dependent ileum.
High-Performance Liquid Chromatography (HPLC)
Chromatographic analysis of CSF samples, both using reversed-phase and gel-filtration techniques reveal distinct fractions, containing a putative "withdrawal substance", which was present only in CSF from the morphine-abstinent animals. In general, distinct UVpatterns (taken at 280, 254 and 214 nm) were observed in CSF from naive, morphinedependent and morphine-abstinent animals. The estimated relative molecular mass of the active component was around 50 kDa and its short retention time on the reversed-phase column suggests the high hydrophobicity.
Discussion
Behaviour
Administration of CSF samples taken from morphine-abstinent rats (donors) into the lateral ventricle of morphine-dependent rats (recipients), precipitated a withdrawal syndrome, which could not be observed in the naive recipient rats. The CSF-induced withdrawal syndrome was less severe, but qualitatively identical to the naloxoneprecipitated withdrawal syndrome. Artificial CSF or CSF from the naive or morphinedependent animals did not induce withdrawal syndrome in no any group of recipient animals. These results confirmed the earlier data (Malin et al., 1987) related to the withdrawal induced by CSF from morphine-abstinent rats.
Visual Evoked Potentials (VEP)
Peak latencies: The CSF taken from the spontaneous morphine-abstinent donor rat induced a decrease of peak latency N3 in morphine-dependent recipient, which indicates a stimulation of central neurotransmission. The CSF-induced decrease of peak latency was less prominent compared to the effect of naloxone. This is a good reflection of similar differences observed in respect to the severity between the withdrawal syndromes induced by CSF and naloxone. The development of N3 component is a result of massive discharge of lateral geniculate units (Bigler, 1975), while the components P2, N2 and P3 represent a diffuse activity between thalamus, midbrain and cortex (Creel et al., 1974). The peaks N3 and P3 reflect an arousal level in the brain (Joseph et al., 1981). A decrease of peak latencies during naloxone- or CSF-precipitated withdrawal suggest an increase of neuronal excitability in the mentioned brain areas.
VEP amplitude values: The CSF taken from the spontaneous morphine abstinent donor rats and naloxone induced a decrease of amplitude values of several peaks (N2-P3 and P3 N3) in the morphine-dependent recipients. Decrease of amplitude values reflects a neuronal depression, which is rather unexpected finding, since naloxone-precipitated withdrawal was described as a state of psychomotor stimulation (Wise and Bozarth, 1987). The naloxone-induced decrease of VEP amplitudes contrasted also to the suggested epilep togenic properties of naloxone, since this opioid receptor antagonist induced an increase of photically evoked discharges in the naive conscious rats (Shearer et al., 1984). However, the possibility that excitation of some inhibitory neurons may lead to depression of other neurons in the visual pathways of morphine-dependent rat, might explain these controver sies. Furthermore, an anticonvulsant effect of naloxone has also been demonstrated (Carter-Snead III and Bearden, 1982).
In vitro experiments
These experiments showed that CSF taken from the spontaneous morphine-abstinent rats failed to induce a contraction of the isolated morphine-dependent guinea-pig ileum. This contrasted to the naloxone-induced contraction of the isolated morphine-dependent guineapig ileum. It further indicates that CSF taken from the spontaneous morphine-abstinent rat does not interfere with opioid receptors. It is of importance to note that these negative results with CSF in vitro, support the idea that the putative CSF withdrawal-precipitating substance is without properties of a competitive opioid receptor antagonist. It seems that the octapeptide F-8-F-NH2 is also without naloxone-like properties (Allard et al., 1989).
High-Performance Liquid Chromatography (HPLC) study
The bioactive component partially isolated in this study, seems to be different from the octapeptide F-8-F-NH2, due to the higher hydrophobicity and much lower molecular mass of the latter (Kivipelto et al., 1989; Labrouche et al., 1993). We can not, however, exclude at this moment, that such a component might bound to a larger protein, affecting its chromatographic and spectral properties. Attempts to determine this factor in CSF of morphine-abstinent rats as well as the examination of bioactivity of these fractions are in progress.
Concluding, this study shows that a "withdrawal substance", not yet chemically defined is synthesized and released in CSF during the development of spontaneous morphine abstinence. This substance is formed in sufficiently high concentrations to induce a withdrawal in morphine-dependent recipient rats. Regarding to the fact that total CSF volume in a 300 g rat is about 580 ul (Lai et al., 1983) and that total CSF volume of rats is replaced completely within 10-25 min (Bouman and Van Wimersma Greidanus, 1979), the release of this substance during withdrawal has to be very abundant. The CSF taken from the spontaneous morphine-abstinent rats decreased the VEP peak latencies and amplitude values, which is identical to the corresponding effects of naloxone in the morphine-dependent rats. However, data from the in vitro study indicate that CSF from spontaneous morphine-abstinent rats does not exert a naloxone-like activity on morphinedependent guinea-pig ileum. More studies are necessary in order to clarify the chemical and bioactive properties of the "withdrawal substance".
References
Aghajanian GK, Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine, Nature 276: 186-188, 1978.
Allard M, Geoffre S, Legendre P, Vincent JD and Simonnet G, Characterization of rat spinal cord receptors to FLFQPQRFamide, a mammalian morphine modulating peptide: a binding study, Brain Res 500: 169-176, 1989.
Bigler ED, Lateral geniculate multiple-unit activity related to metrazol potentiated after-discharges, Electroencephal Clin Neurophysiology 39: 491-497, 1975.
Bouman HJ and Van Wimersma Greidanus TB, A rapid and simple cannulation technique for repeated sampling of cerebrospinal fluid in freely moving rats, Brain Res Bull 4: 575-577, 1979.
Carter-Snead III O and Bearden LJ, The epileptogenic spectrum of opiate agonists, Neuropharmacol 21: 1137-1144, 1982.
Creel D, Dustman RE and Beck EC, Intensity of flash illumination and the visually evoked potential of rats, guinea pigs and cats, Vis Res 14: 725-729, 1974.
Cruz SL, Salazar LA and Villarreal JE, A methodological basis for improving the reliability of measurements of opiate abstinence responses in the guinea-pig ileum made dependent in vitro, J Pharm Meth 25: 329-342, 1991.
Dzoljic MM, Rupreht J, Erdmann W, Stijnen TH, Van Briemen LJ and Dzoljic MR, Behavioral and electrophysiological aspects of nitrous oxide dependence. Brain Res Bull 33: 25-31, 1994.
Joseph R, Forest NM, Fiducia D, Como P and Spiegel S, Electrophysiological and behavioral correlates of arousal, Physiol Psychol 9: 90-95, 1981.
Kivipelto L, Majane EA, Yang HYT and Panula P, Immunohistochemical distribution and partial characterization of FLFQPQRFamidelike peptides in the central nervous system of rats, J Comp Neurol 286: 269-287, 1989.
Labrouche S, Verdot L, Theodosis DT, Allard M and Simonnet G, Characterization of a morphinemodulating peptide, FLFQPQRFamide, in the rat hypophysis: biochemical and immunocytochemical studies, Endocrinology 132: 2191-2198, 1993.
Lai YL, Smith PM, Lamm WJE and Hildebrandt J, Sampling and analysis of cerebrospinal fluid for chronic studies in awake rats, J Appl Physiol 54: 1754-1757, 1983.
Malin DH, Harter L, Jenkins PD, Monfort RD, Bruce PD, Farley PA, Ferebee R, Thrasher KL and Marullo DS, Cerebrospinal fluid from morphine-dependent rats precipitates opiate abstinence syndrome, Life Sci 41: 377-383, 1987.
Malin DH, Lake JR, Hammond MV, Fowler DE, Rogillio RB, Brown SL, Sims JL, Leecraft BM and Yang HY, FMRF-NH 2 like mammalian octapeptide: possible role in opiate dependence and abstinence, Peptides 11: 969-972, 1990a.
Malin DH, Lake JR, Fowler DE, Hammond MV, Brown SL, Leyva JE, Prasco PE and Dougherty TM, FMRF-NH 2 like mammalian peptide precipitates opiate-withdrawal syndrome in the rat, Peptides 11: 277-280,1990b.
Munro AF, Effect of autonomic drugs on the responses of isolated preparations from the guineapig intestine to electrical stimulation, J Physiol (Lond) 120: 41-52, 1953.
Neal BS and Sparber SB, Mianserin attenuates naloxone-precipitated withdrawal signs in rats acutely or chronically dependent upon morphine, J Pharmacol Exp Ther 236: 157-165, 1986. Nyberg F, Lyrenas S and Danielsson A, Fingerprinting of molecular components in individual human cerebrospinal fluid samples with a new micropurification system, J Chromat 548: 311318, 1991.
Paakkari I, A simple method for the verification of a successful cannulation of the rat cerebral ventricles, Experientia 36: 887-889, 1980.
Renlund S, Erlandsson I, Hellman U, Silberring J, Wernstedt C, Lindstrom L and Nyberg F, Micropurification and amino acid sequence of f3-casomorphin-8 in milk from a woman with postpartum psychosis, Peptides 14: 1125-1132, 1993.
Shearer DE, Calder LD, Dustman RE and Snyder EW, Naloxone-induced augmentation of the photically evoked afterdischarge in conscious rats, Brain Res Bull 12: 437-439, 1984.
Wise RA and Bozarth MA, A psychomotor stimulant theory of addiction, Psychol Rev 94: 469492, 1987.
| < Prev | Next > |
|---|












