Chapter 3 Excitatory amino acid receptor antagonists and naloxoneprecipitated withdrawal syndrome in morphine-dependent mice
| Books - Modulators of Drug Dependence Phenomena |
Drug Abuse
Chapter 3 Excitatory amino acid receptor antagonists and naloxoneprecipitated withdrawal syndrome in morphine-dependent mice
Abstract - The effects of excitatory amino acid (EAA) receptor antagonists MK801 (non-competitive NMDA receptor antagonist), DNQX (competitive nonNMDA receptor antagonist) and 5,7-DCKA (antagonist of glycine site of NMDA receptor) have been examined on the naloxone (4 mg/kg, i.p.)-precipitated withdrawal jumping behaviour in morphine-dependent mice. The results indicate that withdrawal jumping behaviour in morphine-dependent mice was attenuated by all three EAA receptor antagonists, MK-801, DNQX and 5,7-DCKA. However, MK-801, DNQX and 5,7 -DCKA inhibited the jumping behaviour in a relatively narrow dose range Published in European Neuropsychopharmacology 3: 111-116, 1993.
Central excitatory amino acids (EAAs) with corresponding receptors have been the focus of much attention in order to clarify neuronal development, long-term potentiation, kindling, epilepsy, learning or memory (Cotman and Iversen, 1987). In addition, there is evidence of the involvement of EAA in drug dependence phenomena. It has been shown that MK-801 blocks alcohol withdrawal seizures in the rat (Morrisett et al., 1990). It seems that chronic alcohol treatment uregulates the number of N-methyl-D-aspartate (NMDA) receptors in the hippocampus (Grant et al., 1990), which might explain both the seizures in alcohol withdrawal and anticonvulsant activity of NMDA receptor antagonists.
Furthermore, recent data indicate that the non-selective antagonist of EAA receptors, kynurenic acid (Krystal et al., 1990) attenuated naloxone-precipitated withdrawal in rats. Similarly, the non-competitive (MK-801) and competitive (LY274614) NMDA antagonists suppressed the behavioural signs of withdrawal in morphine-dependent rats (Koyuncuoglu et al., 1992; Rasmussen et al., 1991; Trujillo and Akil, 1991). Evidently, the functional activity of NMDA receptors may have a modulatory effect on drug dependence phenomena in the rat. The role of other EAA receptors, besides the NMDA receptor, in morphine dependence remains unclarified.
The aim of this study is to examine the role of various EAA receptor antagonists in naloxone-precipitated withdrawal in morphine-dependent mice. We used the non-competitive NMDA receptor antagonist MK-801 (Wong et al., 1988), the non-NMDA receptor antagonist DNQX (Honor6 et al., 1988) and 5,7-DCKA, a selective antagonist of NMDA receptor-associated strychnine-insensitive glycine binding site (Baron et al., 1991). An additional reason for using 5,7-DCKA was that glycine receptor antagonism may produce motor effects different from the competitive NMDA receptor antagonists (Koek and Colpaert, 1990). Materials and Methods
Animals
Male Swiss mice weighing 25-35 g were used in all experiments. The animals were housed singly in polyethylene cages with food and water ad libitum. Artificial light was supplied in a 12-h light-dark cycle.
Morphine dependence
In general, the experimental model for the opioid withdrawal in mice was followed (Way et al., 1969; Kosersky et al., 1974). Chronic morphine dependence in mice was induced by morphine pellets (25 mg morphine base/mouse, s.c.) implanted on the back of the animal under ether anaesthesia. The morphine withdrawal was precipitated by administration of naloxone (4 mg/kg, i.p.), 72 h after the implantation of the pellet. The withdrawal severity was quantified by counting the frequency of jumping from a circular platform (30 cm high, 12 cm diameter). The general behaviour of drug-treated naive and morphine-dependent mice was observed and registered.
The animal was pretreated with vehicle or one EAA receptor antagonist, 30 min prior to naloxone. The pretreated animal was placed on the platform and observed for the following 30 min (in time intervals of 5 min). At the end of the 30-min period, the animal was given naloxone and placed again on the platform in order to be observed in a similar way, for the following 30 min.
Experimental protocol
Morphine-dependent mice were divided into five groups, pretreated intraperitoneally with vehicle (saline, 0.5 ml, n=5; DMSO, 0.5 ml, n=5), MK-801 (1-80 Vg/kg, n=78), DNQX (0.6310 mg/kg, n=40) or 5,7-DCKA (5-160 mg/kg, n=35). In all these animals, the withdrawal jumping behaviour was precipitated by administration of naloxone (4 mg/kg, i.p.), 30 min after drug pretreatment. In order to observe the behavioural effect of EAA antagonist in morphine-dependent mice, naloxone was replaced by saline. These three additional groups (n=14-36) were pretreated (30 min before saline) with various doses of EAA receptor antagonists. Each animal was used only once.
Drugs
The following drugs were used: MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d] cyclohepten 5,10-imine maleate, Research Biochemicals Inc. USA], DNQX (6,7dinitroquinoxaline 2,3-dione, Tocris Neuramin) and 5,7-DCKA (5,7-dichlorokynurenic acid, Brunschwig Chemie). Two compounds, MK-801 and DNQX, were dissolved in distilled water. The pH of MK-801 was adjusted to 7-8, while the pH of DNQX was adjusted to 9 in order to solubilise the compound completely. 5,7-DCKA was dissolved in 10% DMSO (dimethylsulfoxide) and adjusted to pH 7-8. In the control experiments the vehicle solutions were adjusted to the corresponding pH value of drugs. All drug solutions were administered i.p. and given in an equal volume (0.5 ml/injection).
Statistics
Data are expressed as medians. The effects of drug and vehicle treatment were evaluated statistically using the non-parametric Kruskal-Wallis one-way analysis of variance, followed by the Mann-Whitney U-test, with a level of P<0.05 being considered significant (Glantz, 1989).
Results
MK-801
In our preliminary experiments we observed that administration of MK-801, in a dose range of 0.1-10 mg/kg (i.p.), induced a pronounced locomotor dysfunction in both naive and morphine-dependent mice, consisting of wild running (hyperlocomotion), jumping, ataxia and convulsion. The incidence of these locomotor disturbances was dose related, while the higher doses of MK-801 (1-10 mg/kg) induced mainly ataxia and convulsions. However, concentrations of MK-801 below 0.1 mg/kg did not affect a normal behaviour of naive or morphinedependent mice.
In order to avoid the influence of disturbed locomotion on the withdrawal jumping in mice, we used MK-801 in a dose range of 1-80 pg/kg (i.p.) which did not affect locomotion in naive or morphine-dependent mice. MK-801 significantly attenuated the naloxone precipitated jumping behaviour in a dose range of 5-20 pg/kg, i.p. (Fig. 1). The higher doses of MK-801 (40-80 pg/kg, i.p.) were without significant effect on withdrawal jumping behaviour of mice
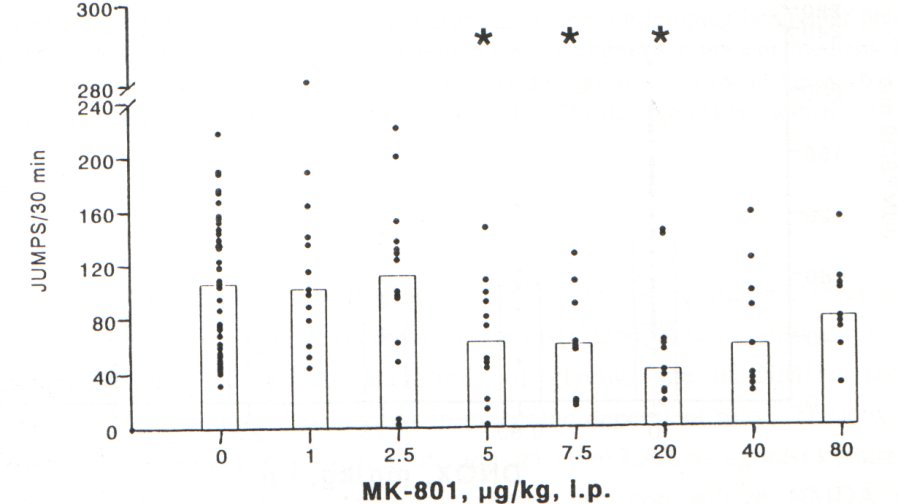
Fig. 1. Effect of MK-801 (pg/kg, i.p., 30 min before naloxone) on jumping behaviour precipitated by naloxone (4 mg/kg, i.p.) in morphine-dependent mice. Histograms represent medians and dots indicate individual animal scores (n=9-14 in MK-801-treated groups, control group = 36). * Significant difference (P<0.05) from the value in vehicle (saline)-pretreated animals.
DNQX
DNQX (20-80 mg/kg, i.p.) caused convulsions (observation period 60 min after drug administration) and/or death (observation period 72 h after drug administration) in morphinedependent mice. However, in naive mice neither convulsions nor death has been observed (Table 1). Therefore, in our further experiments we selected lower concentrations of DNQX (0.63-10 mg/kg, i.p.), which did not induce convulsions in morphine-dependent or naive mice. DNQX reduced naloxone-precipitated withdrawal jumping in mice in a dose range of 1.25-5 mg/kg, i.p. Similarly to MK-801, the dose-response curve of DNQX was also U-shaped (Fig. 2)
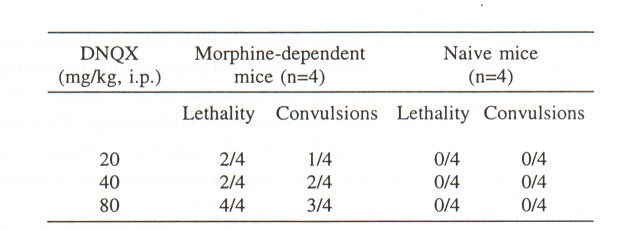
Table 1. Lethal (observation period 72 h after drug administration) and convulsant (observation period 60 min after drug administration) effect of DNQX (20-80 mg/kg, i.p.) on morphine-dependent and naive mice.
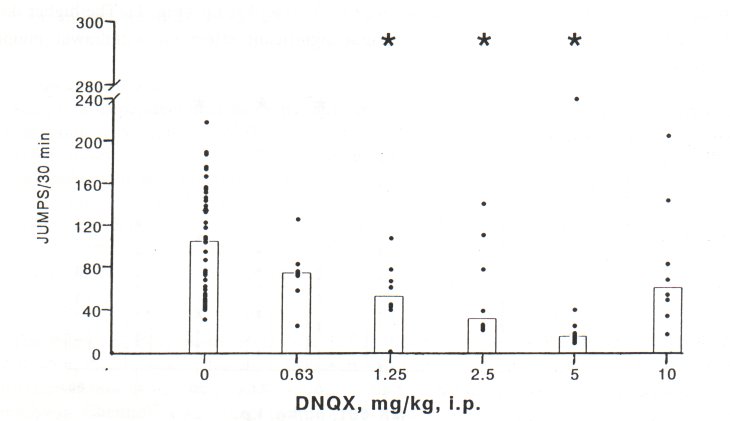
Fig. 2. Effect of DNQX (mg/kg, i.p., 30 min before naloxone) on jumping behaviour precipitated by naloxone (4 mg/kg, i.p.) in morphine-dependent mice. Histograms represent medians and dots indicate individual animal scores (n=6-8 in DNQX-treated groups, control group = 36).* Significant difference (P<0.05) from the value in the vehicle (saline)-pretreated animals
5, 7-DCKA
Administration of 5,7-DCKA in a relatively wide dose range (5-160 mg/kg, i.p.) did not affect the usual behavioral pattern of either naive or morphine-dependent mice. However, 5,7DCKA in a dose range of 20-40 mg/kg, i.p. significantly attenuated the naloxone-precipitated jumping behaviour in morphine-dependent mice (Fig. 3). The maximal effect was seen after treatment with 40 mg/kg 5,7-DCKA, while higher doses (80-160 mg/kg) were ineffective
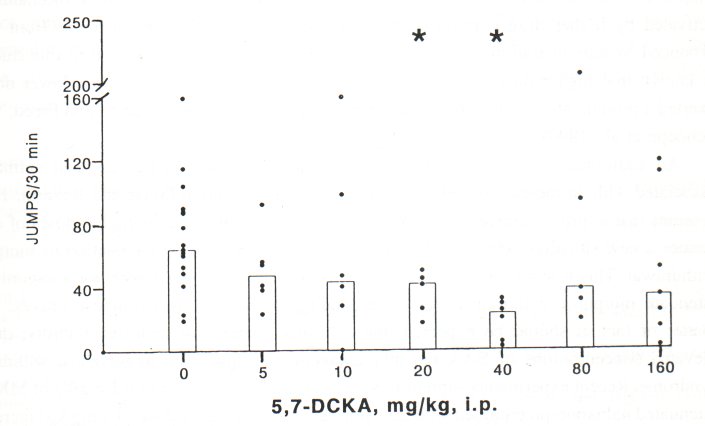
Fig. 3. Effect of 5,7 DCKA (mg/kg, i.p., 30 min before naloxone) on jumping behaviour precipitated by naloxone (4 mg/kg, i.p.) in morphine-dependent mice. Histograms represent medians and dots indicate individual animal scores (n=5-7 in 5,7-DCKA-treated groups, control group = 16). * Significant difference (P<0.05) from the value in vehicle (DMSO)-pretreated animals.
Discussion
Our present findings demonstrate that antagonists of various glutamate receptors, such as MK-801 (non-competitive NMDA receptor antagonist), DNQX (competitive non-NMDA receptor antagonist) and 5,7-DCKA (antagonist of glycine site of NMDA receptors), attenuated the jumping withdrawal behaviour in morphine-dependent mice. These results are consistent with previous works showing that the non-selective EAA antagonist kynurenic acid (Krystal et al., 1990) and selective non-competitive and competitive NMDA receptor antagonists MK-801 and LY274614, respectively, suppressed the withdrawal signs in morphine-dependent rats (Koyuncuoglu et al., 1992; Rasmussen et al., 1991; Trujillo and Akil, 1991). However, in addition to NMDA receptors, this study indicates an involvement of the glycine site of NMDA receptors and non-NMDA receptors in drug withdrawal as well. Each of these three substances has a U-shaped dose-effect curve. Although unusual, a Ushaped dose-effect curve should not be considered as an exceptional phenomenon in the research of drug withdrawal. For example, buprenorphine in lower doses (0.01-0.5 mg/kg) precipitated abstention symptoms in morphine-dependent mice, while the higher doses (1-50 mg/kg) were less active or completely inactive (Lizasoain et al., 1991). The reason for the Ushaped dose effect curve is not known, but it could be assumed that a new mechanism(s) activated by higher drug concentrations may induce effects that are different from those produced by administration of lower doses. As regards the substances used in this study, it is known that higher doses of EAA receptor antagonists (in contrast to the lower doses), exerted a prominent excitatory/proconvulsant effect in naive animals (Jurson and Freed, 1990; Schoepp et al., 1990).
An additional relevant point is that morphine withdrawal in humans and animals is associated with an increase of neuronal and behavioral excitation (Wise and Bozarth, 1987). It seems that a further increase of central neuro-excitability induced by higher doses of drugs creates a new situation, which is presumably not favourable for an attenuation of morphine withdrawal. This might explain a failure of the higher doses of EAA receptor antagonists to attenuate morphine withdrawal and corresponding U-shaped doses-response curves. As a matter of fact, it should be expected that a further increase of neuro-excitability, due to elevated concentrations of EAA receptor antagonists, might even aggravate a withdrawal syndrome. Recent experiments support this idea, since administration of 0.1 mg/kg of MK-801 attenuated naloxone-precipitated withdrawal in rats, while a higher dose (0.3 mg/kg) increased the severity of the same abstention syndrome (Koyuncuoglu et al., 1992).
The mechanism of the suppressing effect of EAA antagonists on morphine withdrawal remains to be elucidated. There is evidence that the noradrenergic system plays an important role in opioid withdrawal. It has been reported that opioid withdrawal is associated with noradrenaline (NA) release (Laverty and Roth, 1980) and increased activity of noradrenergic cells in the locus coeruleus (Aghajanian, 1978; Valentino and Wehby, 1989). Several studies, in vitro and in vivo, indicate that NMDA antagonists may decrease NA release (Jones et al., 1987; Pittaluga and Raiteri, 1992) or activity (Burgard et al., 1989; Dahl and Sarvey, 1990; Loscher et al., 1991). In this respect, it is of importance that MK-801 significantly decreased the levels of NA and adrenaline in the amygdala of naive rats (Loscher et al., 1991). This might be of relevance, since the amygdala was implicated in emotion-related behaviour (Coulombe and White, 1978) and fear response (Hitchcock and Davis, 1986). An anxiolytic activity of NMDA antagonists, observed in naive mice (Trullas et al., 1989) and rats (Kehne et al., 1991), might play a role in the attenuation of withdrawal jumping in mice, since anxiety is a symptom of opioid withdrawal in humans and animals (Lal and Emmett-Oglesby, 1983).
An observation that relatively high doses of DNQX promote convulsions in morphinedependent mice, while the naive mice remained unaffected, deserves a comment. Recent data indicate that morphine dependence is associated with upregulation and/or supersensitivity of NMDA receptors (Marek et al., 1991). Other EAA receptors were not examined. A derangement of these, and possibly other EAA receptors, may explain a different reactivity to EAA receptor antagonists in morphine-dependent and naive mice.
In conclusion, the attenuating effect of EAA receptor antagonists on opioid withdrawal in mice might be due to complex changes in the activity of neurotransmitters and corresponding behavioral alterations in the addicted subjects. For further study the role of the NMDA glycine site is of particular interest, since drugs acting on this site are devoid of muscle-relaxant properties and possess significant anxiolytic effects.
References
Aghajanian GK, Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine, Nature 276: 186-188, 1978.
Baron BM, Siegel BW, Slone AL, Harrison BL, Palfreyman MG and Hurt SD, [3H]5,7Dichlorokynurenic acid, a novel radioligand labels NMDA receptor-associated glycine binding sites, Eur J Pharmacol 206: 149-154, 1991.
Burgard EC, Decker G and Sarvey JM, NMDA receptor antagonists block norepinephrine-induced long-lasting potentiation and long-term potentiation in rat dentate gyrus. Brain Res 482: 351-355, 1989.
Cotman CW and Iversen LL, Excitatory amino acids in the brain-focus on NMDA receptors, Trends Neurochem Sci 10: 263-265, 1987.
Coulombe D and White N, Effects of lesions of the amygdala, pyriform cortex, and stria terminalis on two types of exploration by rats, Physiol Psychol 9: 319-324, 1978.
Dahl D and Sarvey JM, f3-Adrenergic agonist induced long-lasting synaptic modifications in hippocampal dentate gyrus require activation of NMDA receptors, but not electrical activation of afferents, Brain Res 526: 347-350, 1990.
Glantz SA, Alternatives to analysis of variance and the t-test based on ranks, In: Primer of Biostatistics, B Kaufman-Barry and J White (eds.), McGraw-Hill, Singapore, pp. 291-317, 1989. Grant KA, Valverius P, Hudspith M and Tabakoff B, Ethanol withdrawal seizures and the NMDA receptor complex, Eur J Pharmacol 176: 289-296, 1990.
Hitchcock J and Davis M, Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm, Behav Neurosci 100: 11-22, 1986.
Honore T, Davies SN, Drejer J, Fletcher EJ, Jacobsen P, Lodge D and Nielsen FE, Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists, Science 241: 701-'703, 1988. Jones SM, Snell LD and Johnson KM, Phencyclidine selectively inhibits N-methyl-D-aspartate induced hippocampal [3H]norepinephrine release, J Pharmacol Exp Ther 240: 492-497, 1987.
Jurson PA and Freed WJ, A slight anticonvulsant effect of CNQX and DNQX as measured by homocysteine- and quisqualate-induced seizures, Pharmacol Biochern Behav 36: 177-181, 1990. Kehne JH, McCloskey TC, Baron BM, Chi EM, Harrison BL, Whitten JP and Palfreyman MG, NMDA receptor complex antagonists have potential anxiolytic effects as measured with separation-induced ultrasonic vocalizations, Eur J Phannacol 193: 283-292, 1991.
Koek W and Colpaert FC, Selective blockade of N-methyl-D-aspartate (NMDA)-induced convulsions by NMDA antagonists and putative glycine antagonists: relationship with phencyclidine-like behavioral effects, J Pharmacol Exp Ther 252: 349-357, 1990.
Kosersky DS, Harris RA and Harris LS, Naloxone-precipitated jumping activity in mice following the acute administration of morphine, Eur J Pharmacol 26: 122-124, 1974.
Koyuncuoglu H, Dizdar Y, Aricioglu F and Sayin U, Effects of MK-801 on morphine physical dependence: Attenuation and intensification, Pharmacol Biochem Behav 43: 487-490, 1992. Krystal JH, Rasmussen K and Aghajanian GK, Excitatory amino acid antagonists for opiate withdrawal, Am Psych Assoc Abstr 143: 262, 1990.
Lal H and Emmett-Oglesby MW, Behavioral analogues of anxiety. Animal models, Neuropharmacology 22: 1423-1441, 1983.
Laverty R and Roth RH, Clonidine reverses the increased norepinephrine turnover during morphine withdrawal in rats, Brain Res 182: 482-485, 1980.
Lizasoain I, Leza JC and Lorenzo P, Buprenorphine: bell-shaped dose-response curve for its antagonist effects, Gen Pharmacol 22: 297-300, 1991.
Loscher W, Armies R and Honack D, The N-Methyl-D-Aspartate receptor antagonist MK-801 induces increases in dopamine and serotonin metabolism in several brain regions of rats, Neurosci Lett 128: 191-194,1991.
Marek P, Ben-Eliyahu S, Gold M and Liebeskind JC, Excitatory amino acid antagonists (kynurenic acid and MK-801) attenuate the development of morphine tolerance in the rat, Brain Res 547: 77-81,1991.
Morrisett RA, Rezvani AH, Overstreet D, Janowsky DS, Wilson WA and Swartzwelder HS, MK-801 potently inhibits alcohol withdrawal seizures in rats, Eur J Pharmacol 176: 103-105, 1990. Pittaluga A and Raiteri M, N-methyl-D-asparate (NMDA) and non-NMDA receptors regulating hippocampal norepinephrine release. 1. Location on axon terminals and pharmacological characterization, J Pharmacol Exp Ther 260: 232-237, 1992.
Rasmussen K, Fuller RW, Stockton ME, Perry KW, Swinford RM and Ornstein PL, NMDA receptor antagonists suppress behaviors but not norepinephrine turnover or locus coeruleus unit activity induced by opiate withdrawal, Eur J Pharmacol 197: 9-16, 1991.
Schoepp DD, Gamble AY, Salhoff CR, Johnson BG and Omstein PL, Excitatory amino acid-induced convulsions in neonatal rats mediated by distinct receptor subtypes, Eur J Pharmacol 182: 421427, 1990.
Trujillo KA and Akil H, Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801, Science 251: 85-87, 1991.
Trullas R, Jackson B and Skolnick P, Anxiolytic properties of 1-aminocyclopropane-carboxylic acid, a ligand at strychnine-insensitive glycine receptors, Pharmacol Biochem Behav 34: 313-316, 1989. Valentino RJ and Wehby RG, Locus ceruleus discharge characteristics of morphine-dependent rats: Effects of naltrexone, Brain Res 488: 126-134, 1989.
Way EL, Loh HH and Shen F, Simultaneous quantitative assessment of morphine tolerance and physical dependence, J Pharmacol Exp Ther 167: 1-8, 1969.
Wise RA and Bozarth MA, A psychomotor theory of addiction, Psychological Rev 94: 469-492, 1987.
Wong EHF, Knight AR and Woodruff GN, [3H]MK-801 labels a site on the N-methyl-D-aspartate receptor channel complex in rat brain membranes, J Neurochem 50: 274-281, 1988
| < Prev | Next > |
|---|












