Chapter 2 Opioids
| Books - Modulators of Drug Dependence Phenomena |
Drug Abuse
Chapter 2 Opioids
2.1. Opioids and opioid receptors
The opioids includes four different groups of compounds:
"True opiates" natural alkaloids derived from the opium poppy (papaver somniferum), such as morphine and codeine.
• Semi-synthetic opioids, structurally related to morphine (heroine).
• Synthetic opioids, structurally unrelated to morphine (fentanyl, methadone, pentazocine, etc).
• Endogenous opioid peptides (beta-endorphin, Met- and Leu-enkephalin, dynorphin A and B) were identified (Li et al., 1976; Hughes et al., 1975; Goldstein et al., 1979; Cone and Goldstein, 1982, respectively), following the discovery of stereospecific opioid binding site in the CNS (Pert and Snyder, 1973; Simon et al., 1973; Terenius, 1973).
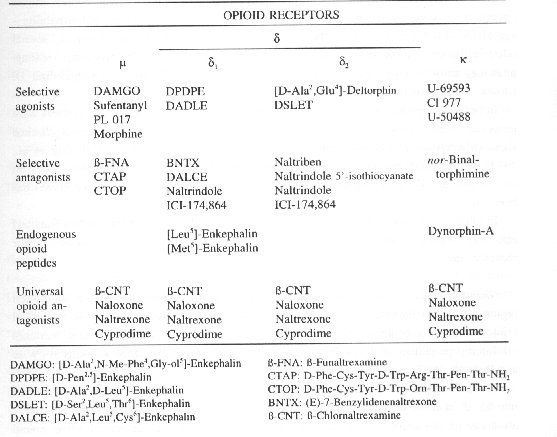
Opioid receptors are distributed throughout the mammalian CNS (Atweh and Kuhar 1977a,b,c), but could also be found in periphery (Cox, 1988). Three main receptor types were identified N, x, and S (Martin et al., 1976; Lord et al., 1977; Chang et al., 1979). Recent studies have demonstrated the existence of two 8-opioid receptor subtypes (8, and 82) (Jiang et al., 1991). Table 1. shows the proposed classification of opioid receptors with corresponding agonists and antagonists.
2.2. Opioid dependence
Neuronal pathways and neurotransmitters. It is claimed that psychic dependence to opioids and many other drugs is regulated by three main anatomically well-defined brain areas (Koob, 1992). These areas are the followings: 1. ventral tegmental area (VTA), in which the cell bodies of the mesocorticolimbic dopamine (DA) system originate, 2. nucleus accumbens (NAc), which receives projections from the VTA, 3. ventral pallidum, which receives a major projection from the NAc.
Experimental studies showed that rats will self-administer opioids into the VTA, while opioid peptides injected into this brain region produce place preference (Di Chiara and North, 1992).
It has been shown that microinjection of the neurotoxin kainic acid, which destroys the cell bodies, but not fibres of passage, into the NAc markedly decreased an intravenous self-administration of both opioids and psychostimulants (Zito et al., 1985).
A similar effect was observed in the ventral pallidum following selective destruction of cell bodies by ibotenic acid (Hubner and Koob, 1987).
It was claimed that the DA neurons of the VTA are critical for opioid reinforcement (Bozarth and Wise, 1984). It seems that opioids excite DA neurons in the VTA, via preceptors located on GABA (y-amino butyric acid)-releasing neurons.
Opioids can induce a hyperpolarization of these GABA- neurons, by increasing the K' efflux. As a result, GABA release onto the DA cells is reduced and the firing rate of DA neurons is increased (Johnson and North, 1992; Fig.1). It is proposed that p- and -receptors are implicated in mediating the reinforcing actions of opioids, while x-receptors mediate their aversive actions (Di Chiara and Imperato, 1988; Spanagel et al., 1990).
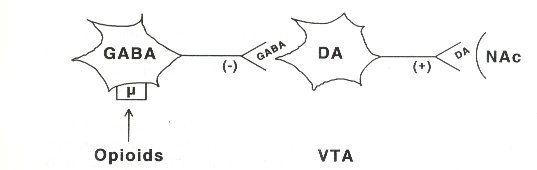
Fig. 1. Schematic illustration of the way in which DA-containing neurons in the ventral tegmental area (VTA) are excited by opioids. GABA-containing interneurons are hyperpolarized by opioids acting at p-receptors. This results in decreased (-) GABA release and increased (+) firing and DA release of DA-containing neurons in the VTA towards the nucleus accumbens (NAc).
The level of other neurotransmitters, besides DA, is also deranged during opioid dependence. Several studies have been demonstrated that the excitatory amino acid (EAA) receptor system is involved in the process of opioid dependence. Morphine is known to inhibit the enzymes producing aspartic acid and glutamic acids (Koyuncuoglu et al., 1979, 1986) from asparagine and glutamine, respectively (Bielarczyk et al., 1986), resulting in the decreased level of EAAs. Accordingly, the chronic presence of opioid receptor agonists decreases a normal activation of NMDA receptors (Aanonsen and Wilcox, 1987; Tanganelli et al., 1991). Therefore, morphine dependence is associated with NMDA receptor up-regulation and/or supersensitivity (Koyuncuoglu et al., 1992a,b).
2.3. Withdrawal syndrome
Cessation of opioid agonist or administration of opioid receptor antagonist in opioiddependent subjects induces a withdrawal syndrome. Although physical dependence occurs mainly following chronic exposure to an opioid drug, a withdrawal syndrome can be precipitated in man (Bickel et al., 1988) and various animals (Martin and Eades, 1961;Way et al., 1969; Meyer and Sparber, 1977; Krystal and Redmond, 1983), following an acute opioid treatment as well.
Opioid physical dependence can be easily studied, since opioid withdrawal syndrome, induced by diverse opioid antagonists (Table 1.), can be abruptly abolished by opioid agonists (Wei et al., 1973a,b).
Table l. Opioid receptor classification and corresponding drugs, which interfere with these receptors (adapted from The RBI handbook of receptor classification, by Kebabian and Neumeyer (eds.), 1994)
Behaviour. Heroin or morphine have in men a short half-life (2 to 3 h). The onset of withdrawal symptoms occurs within 8 to 16 h after the last dose, and the peak effect is around 2-3 days. Methadone has a longer half-life (15-20 h) and the onset of withdrawal symptoms is within 2-3 days after the last use. However, the peak effect is around 1-2 weeks, and some symptoms persist for months before resolution occurs (Zweben and Payte, 1990). It has been demonstrated a long time ago (Himmelsbach et al., 1938, cited by Martin and Sloan, 1977) that the opioid withdrawal symptoms - nausea, vomiting, sweating, gooseflesh, diarrhoea, tremor, chills and fever - occurred predictably by discontinuing morphine administration in a person who had been maintained on a regular schedule for morphine injections with escalating dosage. Himmelsbach et al., (1938, cited by Martin and Sloan, 1977) developed a method of scoring the intensity of the withdrawal syndrome, placing emphasis on easily recognized objective disturbances rather than on subjective complaints.
The withdrawal syndrome in morphine-dependent rats includes whole-body shakes ("wet-dog" shakes), diarrhoea, escape jumps, teeth-chattering, salivation and irritability - aggression (Martin et al., 1963; Blasig et al., 1973; Wei et al., 1973a). Later on, several other withdrawal signs have been specified, for example sniffing, grooming, rearing, gnawing, penile-licking, mastication, ptosis, writhing and rhinorrhoea (Acquas and Di Chiara, 1992; Maldonado and Koob, 1993; Gold et al., 1994).
In order to classify the severity of withdrawal syndrome, several scoring systems were proposed. Some researchers divided the withdrawal symptoms in counted and checked signs (Maldonado and Koob, 1993), or in dominant and recessive ones (Blasig et al., 1973), while others provided signs with a weighting factor (Neal and Sparber, 1986). In our studies, described in chapters 5-7, the scoring system of Neal and Sparber (1986) has been used.
In table 2., are listed several withdrawal signs in morphine-dependent animals (rats and mice) in respect to their origin and involvement of specific neurotransmitters and/or receptor sites.
Neuronal pathways and neurotransmitters. The locus coeruleus (LC) is the main brain region playing an important role in the opioid withdrawal syndrome, but less in the opioid reinforcement. In contrast, the mesolimbic dopaminergic (DA-ergic) system mediates reinforcing properties of drugs, but is not extensively involved in drug withdrawal (Wise and Bozarth, 1987).
The LC is located on the floor of the fourth ventricle in the anterior pons. The small number of neurons provides widespread noradrenergic (NA-ergic) innervation to virtually all areas of the brain and spinal cord. Destruction of the LC decreased some opioid withdrawal signs, such as chewing and rearing in morphine-dependent rats (Maldonado and Koob, 1993). However, LC neurons recorded in slices from morphine-dependent rats do not exhibit a pronounced withdrawal hyperactivity (Christie et al., 1987), indicating that most of the withdrawal-induced activation of these cells observed in vivo is likely to be mediated by afferents to the LC. Studies revealed that the rostral medullary nucleus paragigantocellularis is the major excitatory input to LC neurons, acting primarily viaEAAs (Ennis and Aston-Jones, 1988; Hong et al., 1993).
The LC contains a high density of opioid receptors and it receives substantial direct enkephalin inputs. f3-Endorphin and dynorphin fibres are found in the LC area (AstonJones et al., 1993). Presynaptic opioid receptors located on terminals of central NA-ergic neurons, are probably responsible for the decreased release of NA (Arbilla and Langer, 1978) and the diminution of the LC terminal excitability that follows opioids exposure (Nakamura et al., 1982). In vitro studies revealed that opioids acting at N receptor may increase K+ efflux and inhibit Na' influx, which is followed by hyperpolarization of the LC neurons (Andrade et al., 1983).
Clonidine (beta2-agonist), a drug that decreases NA-ergic activity, blocks both opioid withdrawal symptoms and behaviour induced byelectrical stimulation of the LC (Maldonado and Koob, 1993). It has been shown that clonidine, similarly to opioids, elicit a hyperpolarization in LC neurons (Aghajanian, 1978). Coapplication of clonidine and opioid agonists shows a response similar to that evoked by either agonist alone (Aghajanian and Wang, 1987). This finding implicates that both the obeta2-adrenoceptor agonist andopioid agonists may affect K+ efflux in the same way.
Recently, it has been demonstrated that K'-channel openers can mimic the effects of morphine on neuronal K+ currents, and as a consequence can act as substituents for morphine during withdrawal process (Robles et al., 1994).
Second messenger systems
Acute opioid action (Fig. 2). Opioid-induced inhibition of LC neurons via increasing the conductance of a K+ channel and inhibition of a Na+-dependent inward current (Aghajanian and Wang, 1987) is mediated by the pertussis toxin-sensitive G-proteins (Blume, 1978). Administration of opioids leads to activation of the K+ channel by direct coupling of the opioid receptor to the K+ channel via a G-protein. In contrast, inhibition of the Na'-dependent current appears to be indirect. Namely, the Na+ current is normally activated by a cAMP (cyclic adenosine monophosphate)-dependent protein kinase, either through direct phosphorylation of the Na' channel or by phosphorylation of some associated proteins (Wang and Aghajanian, 1990). The opioid inhibition of the NA' current appears to be mediated via inhibition of adenylate cyclase (AC) and reduced levels of cAMP. Reduced levels of CAMP decrease cAMP-dependent protein kinase activity and phosphorylation of the responsible channel/pump or closely related associated proteins. In addition to reduced firing rates (due to hyperpolarization), inhibition of cAMP pathway decreases catecholamine synthesis via reduced phosphorylation of tyrosine hydroxylase. Biochemical studies have confirmed that opioids inhibit AC activity in the LC (Duman et al., 1988) and CAMP-dependent protein phosphorylation (Guitart and Nestler, 1989).
Table 2. Some withdrawal signs observed in rats and/or mice in respect to their origin (central) and the involved neurotransmitter/receptor sites.

1. Adams et al., Physiol & Behav 53: 1127, 1993; 2. Argiolas et al., Reg Pept 45: 139, 1993; 3. Ball et al., Physiol Behav 13: 123, 1974; 4. Baumeister et al., Neuropharmacol 28: 1151, 1989; 5. Baumeister et al., Neuropharmacol 31: 835, 1992; 6. Bedard and Pycock, Neuropharmacol 16: 663, 1992; 7. Bozarth and Wise, Science 224: 516, 1984; 8. Calvino et al., Brain Res 177: 19, 1979; 9. Gunne et al., Psychopharmacol 134, 1972; 10. Jones et al., Brain Res 560: 163, 1991; 11. Krane et al., New Eng Med 321: 1648, 1989; 12. Lammers et al., Brain Res 449: 294, 1988; 13. Lammers et al., Brain Res 449: 311, 1988; 14. Levin et al., Pharmacol Biochem Behav 34: 43, 1989; 15. Maldonado et al., J Pharmacol Exp Ther 261: 669, 1992; 16. Maldonado et al., Neuropharmacol 31: 1231, 1992; 17. Maldonado and Koob, Brain Res 605: 128, 1993; 18. Neal and Sparber, J Pharmacol Exp Ther 236: 157, 1986; 19. Patrick et al., Eur J Pharmacol 231: 243, 1993; 20. Pei et al., Eur J Pharmacol 230: 63, 1993; 21. Pothos et al., Brain Res 566: 348, 1991; 22. Stinus et al., Neurosci 37: 767, 1990; 23. Tremblay and Charton, Neurosci Lett 23: 137, 1981; 24. Tseng et al., Neuropharmacol 14: 247, 1975; 25. Ukai et al., Brain Res 557: 77, 1991; 26. Van Wimersma Greidanus et al., Ear J Pharmacol 173: 227, 1989; 27.Wei et al., J Pharmacol Exp Ther 185: 108, 1973; 28. Yukhananov et al., INRC-abstract p41, 1994.
Chronic opioid action. Following chronic opioid administration, the LC neurons develop tolerance to the prior described acute inhibitory actions as neuronal firing rates recover toward control levels (Aghajanian, 1978; Christie et al., 1987). It was suggested that during chronic exposure to opioids, long term adaptations in intracellular messenger proteins could occur, which could be involved in the process of cellular tolerance, dependence and withdrawal. It has been shown that chronic administration of opioids increases the levels of G-proteins (Nestler et al., 1989) and stimulates the AC (Duman et al., 1988), cAMP-dependent protein kinase (Nestler and Tallman, 1988) in the neurons of the LC. Tyrosine hydroxylase is also activated (Guitart et al., 1990), which is the ratelimiting enzyme, involved in the biosynthesis of catecholamine neurotransmitters (Fig. 2).
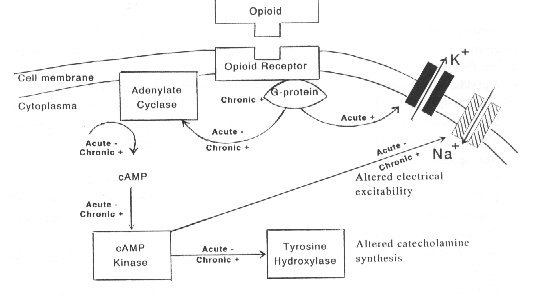
Fig. 2. Opioid actions in the locus coeruleus (LC). Acute administration of opioids inhibited (-) both adenylate cyclase and CAMP-dependent protein phosphorylation. Chronic administration of opioids increased (+) the levels of G-proteins and stimulated (+) the activity of adenylate cyclase, cAMP-dependent protein kinase and tyrosine hydroxylase.
In the chronic opioid-dependent state, the combined presence of opioids and the upregulated cAMP pathway would return the LC firing rate to control levels. Removal of the opioids would leave the up-regulated cAMP pathway unopposed, leading to withdrawal excitation of neuronal activity. Excitation of the LC neurons during withdrawal is necessary for producing many of the behavioral signs of opioid abstinence (Rasmussen et al., 1990; Maldonado and Koob, 1993).
In conclusion, all these findings indicate that upregulation of the cAMP pathway is a likely mechanism of opioid dependence in the LC. It is probably not the only mechanism, but this up-regulated cAMP pathway represent one of the examples in which a behavioral component (physical opioid dependence) can be correlated with biochemical and electrophysiological adaptations occurring in the neurons of the LC.
Following the chronic exposure to opioids, alterations on for example the molecular level (gene expression) have demonstrated (Nestler et al., 1993). However, these changes are not discussed in this thesis.
Treatment of opioid withdrawal syndrome. The expression of the morphine withdrawal syndrome in men/animals could be inhibited by opioids and non-opioids. Some examples are the followings:
• Opioids. High doses (30 fold higher) of opioids terminated the precipitated withdrawal stimulus in animals (Holtzman, 1985) and men (Zweben and Payte, 1990), while the administration of enkephalinase inhibitors attenuated the expression of morphine withdrawal behaviour in rats and mice (Dzoljic, 1986; Dzoljic et al., 1986).
• Non-opioids.
- Clonidine is effective in the treatment of the opioid withdrawal in humans (Gold et al., 1978; Kleber et al., 1980). In morphine-dependent rats, clonidine eliminated "wet-dog" shakes, diarrhoea and teeth-chattering and prevented the release of DA in the NAc (Romandini et al., 1984; Pothos et al., 1991).
- 5-HT Reuptake blockers (d-fenfluramine) attenuated opioid withdrawal jumping in rats (Cervo et al., 1981), a sign which is not influenced by clonidine.
- K'-Channel openers (cromakalim and diazoxide) can mimic the effects of morphine on neuronal K` currents, and could act as substituents for morphine in the withdrawal syndrome (Robles et al., 1994).
- Ca'-Channel blockers (verapamil, nimodipine, flunarizine) reduced several signs of naloxone-precipitated withdrawal such as diarrhoea, ptosis and jumping in morphinedependent rats (Bongianni et al., 1986; Baeyens et al., 1987).
- EAA receptor antagonists (discussed in chapter 3).
- Nitric oxide synthase (NOS) inhibitors (discussed in chapters 4-5).
- lbogaine and norharman (discussed in chapter 7)
2.4. Tolerance
Biochemical changes following tolerance
Opioid receptor system. It has been suggested that chronic opioid treatment alters the opiate receptor density in CNS (Collier, 1965). However, this subject is controversial. Some authors have reported a decrease in the number of p binding sites in tolerant animals (Rogers and El-Fakahany, 1986; Bhargava and Gulati, 1990; Abdelhamid and Takemori, 1991), while others showed no changes (Klee and Streaty, 1974; Nishino et al., 1990) or even an increase of p-receptor binding sites (Pert and Snyder, 1976; Brady et al., 1989).
Second messenger systems. There seemed to be some common mechanism underlying dependence (discussed on page 41) and tolerance. The up-regulated CAMP system likely contributes to tolerance by making it more difficult for opioids to inhibit cAMP system and corresponding increase of the Na'-dependent inward current. It is also possible that the upregulated cAMP system could result in greater levels of opioid receptor density through phosphorylation of the receptor. This hypothesis is based on observations that brief exposure to met-enkephalin desensitizes the p-opioid receptor in the LC and the evidence that agents which activate the cAMP pathway promote this desensitization (Harris and Williams, 1991). By promoting desensitization, the upregulated cAMP system in the tolerant state could lead to a reduced ability of opioids to activate acutely Gproteins and the K' channel. In rats chronically treated with morphine, p-receptors couple less well to G-proteins (Christie et al., 1987; Tao et al., 1993). This uncoupling of receptors and G-proteins may also contribute to the occurrence of opioid tolerance.
References
Aanonsen LM and Wilcox GL, Nociceptive action of excitatory amino acids in the mouse: Effects of spinally administered opioids, phencyclidine and sigma agonists, J Pharmacol Exp Ther 243: 9-19, 1987.
Abdelhamid EE and Takemori AE, Characteristics of mu and delta- opioid binding sites in striatal slices of morphine-tolerant and -dependent mice, Eur J Pharmacol 198: 157-163, 1991.
Acquas E and Di Chiara G, Depression of mesolimbic dopamine transmission and sensitization to morphine during opiate abstinence, J Neurochem 58: 1620-1625, 1992.
Aghajanian GK, Tolerance of locus coeruleus neurons to morphine and suppression of withdrawal response by clonidine, Nature 267: 186-188, 1978.
Aghajanian GK and Wang YY, Common alpha 2- and opiate effector mechanisms in the locus coeruleus: intracellular studies in brain slices, Neuropharmacol 26: 793-799, 1987.
Andrade R, Vandermaelen CP and Aghajanian GK, Morphine tolerance and dependence in the locus coeruleus: single cell studies in brain slices, Eur J Pharmacol 91: 161-168, 1983.
Arbilla S and Langer SZ, Morphine and beta-endorphin inhibit release of noradrenaline from cerebral cortex but not of dopamine from rat striatum, Nature 271: 559-564, 1978.
Aston-Jones G, Shiekhattar R, Akaoka H, Rajkowski J and Kubiak P, Opiates influence locus coeruleus neurons by potent indirect and direct actions, In: The neurobiology of opiates, RP Hammer (ed.), CRC Press, USA, pp. 175-202, 1993.
Atweh SF and Kuhar MJ, Autoradiographic localization of opiate receptors in rat brain. 1. Spinal cord and lower medulla, Brain Res 124: 53-67, 1977a.
Atweh SF and Kuhar MJ, Autoradiographic localization of opiate receptors in rat brain. II. The brainstem, Brain Res 129: 1-12, 1977b.
Atweh SF and Kuhar MJ, Autoradiographic localization of opiate receptors in rat brain. III. The telencephalon, Brain Res 134: 393-405, 1977c.
Baeyens JM, Esposito E, Ossowska G and Samanin R, Effects of peripheral and central administration of calcium channel blockers in the naloxone-precipitated abstinence syndrome in morphine-dependent rats, Ear J Pharmacol 137: 9-13, 1987.
Bhargava FIN and Gulati A, Down-regulation of brain and spinal cord p-opiate receptors in morphine tolerant-dependent rats, Eur J Pharmacol 199: 305-311, 1990.
Bickel WK, Stitzer ML, Liebson IA and Bigelow GE. Acute physical dependence in man: effects of naloxone after brief morphine exposure, J Pharmacol Exp Ther 244: 126-132, 1988. Bielarczyk H, Lysiak W and Szutowicz A, Synthesis of glutamate and aspartate in rat brain synaptosomes, Acta Biochim Pol 33: 239-251, 1986.
Blasig J, Herz A, Reinhold K and Zieglgansberger S, Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats, Psychopharmacologia 33: 19-38, 1973.
Blume AJ, Interaction of ligands with the opiate receptors of brain membranes: regulation by ions and nucleotides, Proc Natl Acad Sci USA 75: 1713-1717, 1978.
Bongianni F, Carla V, Moroni F and Pellegrini-Giampietro DE, Calcium channel inhibitors suppress the morphine withdrawal syndrome in rats, Br J Pharmacol 88: 561-567, 1986. Bozarth MA and Wise RA, Anatomically distinct opiate receptor fields mediate reward and physical dependence, Science 224: 516-517, 1984.
Brady LS, Herkenham M, Long JB and Rothman RB, Chronic morphine increases mu-opiate receptor binding in a rat brain: a quantitative autoradiographic study, Brain Res 477: 382-386, 1989.
Cervo L, Rochat C, Romandini S and Samanin R, Evidence of a preferential role of brain serotonin in the mechanisms leading to naloxone-precipitated compulsive jumping in morphine-dependent rats, Psychopharmacology 74: 271-274, 1981.
Chang K-J, Cooper BR, Hazum E and Cuatrecasas P, Multiple opiate receptors: different regional distribution in the brain and differential binding of opiates and opioid peptides, Mol Pharmacol 16: 91-104, 1979.
Christie MJ, Williams JT and North RA, Cellular mechanisms of opioid tolerance: studies in single brain neurons, Mol Pharmacol 32: 633-638, 1987.
Collier HOJ, A general theory of the genesis of drug dependence by induction of receptors, Nature 205: 181-182, 1965.
Cone RI and Goldstein A, A specific radioimmunoassay for the opioid peptide dynorphin B in neural tissues, Neuropeptides 3: 97-106, 1982.
Cox BM, Peripheral actions mediated by opioid receptors, In: The Opiate Receptors, GW Pasternak (ed.), The Humana Press, Clifton New Jersey, pp. 357-380, 1988.
Di Chiara G and Imperato A, Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats, Proc Natl Acad Sci USA 85: 5274-5278, 1988.
Di Chiara G and North RA, Neurobiology of opiate abuse, Trends Pharmacol Sci 13: 185-193, 1992.
Duman RS, Tallman JF and Nestler EJ, Acute and chronic opiate-regulation of adenylate cyclase in brain: specific effects in locus coeruleus, J Pharmacol Exp Ther 246: 1033-1039, 1988
Dzoljic MR, Enkephalinase inhibitors attenuate naloxone-precipitated withdrawal syndrome, NIDA Res Monogr 75: 575-578, 1986.
Dzoljic MR, Rademaker B, Poel-Heisterkamp AL vd, Upknownwan OE and Haffmans J, Enkephalinase inhibition suppresses naloxone-induced jumping in morphine-dependent mice, Arch Int Pharmacodyn 283: 222-228,1986.
Ennis M and Aston-Jones G, Activation of locus coeruleus neurons from nucleus paragigantocellularis: a new excitatory amino acid pathway in brain, J Neurosci 8: 3644-3657, 1988. Gold MS, Redmond DE Jr and Kleber HD, Clonidine in opiate withdrawal, Lancet 1: 929-933, 1978.
Gold LH, Stinus L, Inturrisi CE and Koob GF, Prolonged tolerance, dependence and abstinence following subcutaneous morphine pellet implantation in the rat, Eur J Pharmacol 253: 45-51, 1994.
Goldstein A, Tachibana S, Lowney LI, Hunkapiller M and Hood L, Dynorphin-(1-13), an extraordinarily potent opioid peptide, Proc Natl Acad Sci USA 76: 6666-6670, 1979.
Guitart X and Nestler EJ, Identification of morphine- and cyclic AMP-regulated phosphoproteins (MARPPs) in the locus coeruleus and other regions of the rat brain: regulation by acute and chronic morphine, J Neurosci 9: 4371-4387, 1989.
Guitart X, Hayward M, Nisenbaum LK, Beitner-Johnson D, Haycock JW and Nestler EJ, Identification of MARPP-58, a morphine- and cyclic AMP-regulated phosphoprotein of 58 kDa, as tyrosine hydroxylase: evidence for regulation of its expression by chronic morphine in the rat locus coeruleus, J Neurosci 10: 2649-2659, 1990.
Harris GC and Williams JT, Transient homologous p-opioid receptor desensitization in rat locus coeruleus neurons, J Neurosci 11: 2574-2581, 1991.
Hong, M, Milne B and Jhamandas K, Evidence for the involvement of excitatory amino acid pathways in the development of precipitated withdrawal from acute and chronic morphine: an in vivo voltammetric study in the rat locus coeruleus, Brain Res 623: 131-141, 1993.
Holtzman SG, Discriminative stimulus effects of morphine withdrawal in the dependent rat: suppression by opiate and nonopiate drugs, J Pharm Exp Ther 233: 80-86, 1985.
Hubner CB and Koob GF, The ventral pallidum plays a role in mediating cocaine and heroin in self-administration in the rat, Brain Res 508: 20-29, 1987.
Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA and Morris HR, Identification of two related pentapeptides from the brain with potent opiate agonist activity, Nature (London) 258:577-579,1975.
Jiang Q, Takemori AE, Sultana M, Portoghese PS, Bowen WD, Mosberg HI and Porreca F, Differential antagonism of opioid delta antinociception by [D-Ala',Leu5,Cys6I enkephalin and naltrindole 5'-isothiocyanate: evidence for delta receptor subtypes. J Pharmacol Exp Ther 257: 1069-1075,1991.
Johnson SW and North RA, Opioids excite dopamine neurons by hyperpolarization of local interneurons, J Neurosci 12: 483-488, 1992.
Kleber HD, Gold MS and Riordan CE, The use of clonidine in detoxification from opiates, Bull Narc 32: 1-10, 1980.
Klee WA and Streaty RA, Narcotic receptor sites in morphine-dependent rats, Nature 248: 61-63, 1974.
Koob GF, Drugs of abuse: anatomy, pharmacology and function of reward pathways, Trends Pharmacol Sci 13: 177-184, 1992.
Koyuncuoglu H, Keyer-Uysal M, Berkman K, Giing6r M and Genc E, The relationship between morphine, aspartic acid and L-asparaginase in rats, Ear J Pharmacol 60: 369-372, 1979. Koyuncuoglu H, Giingbr M, Enginar N, Hatipoglu I and Hizal A, brain asparaginase, AChE activity and plasma cortisol level in morphine dependent rats: Effect of aspartic acid and naloxone, Pharmacol Biochem Behav 25: 953-957, 1986.
Koyuncuoglu H, Uresin Y, Esin Y and Aricioglu F, Morphine and naloxone act similarly on glutamate-caused guinea pig ileum contraction, Pharmacol Biochem Behav 43: 479-482, 1992a. Koyuncuoglu H, Dizdar Y, Aricioglu F and Sayin U, Effects of MK801 on morphine physical dependence: attenuation and intensification, Pharmacol Biochem Behav 43: 487-490, 19926. Krystal JH and Redmond DE, A preliminary description of acute physical dependence on morphine in the vervet monkey, Pharmacol Biochem Behav 18: 289-291, 1983.
Li CH, Lemaine S, Yamashiro D and Doneen BA, The synthesis and opiate activity of betaendorphin, Biochem Biophys Res Commun 71: 19-25, 1976.
Lord JH, Waterfield AA, Hughes J and Kosterlitz HW, Endogenous opioid peptides: Multiple agonists and receptors, Nature 267: 495-499, 1977.
Maldonado R and Koch GF, Destruction of the locus coeruleus decreases physical signs of opiate withdrawal, Brain Res 605: 128-138, 1993.
Martin WR and Eades CG, Demonstration of tolerance and physical dependence in the dog following a short-term infusion of morphine, J Pharmacol Exp Ther 133: 262-270, 1961. Martin WR, Wikler A, Eades CG and Peskor FT, Tolerance to and physical dependence on morphine in rats, Psychopharmacol 4: 247-249, 1963.
Martin WR, Eases CG, Thompson JA, Huppler RE, Gilbert PE, The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog, J Pharmacol Exp Ther 197: 517-532, 1976.
Martin WR and Sloan JW, Neuropharmacology and neurochemistry of subjective effects, analgesia, tolerance and dependence produced by narcotic analgesics, In: Handb Exp Pharmacol, R Hoffmeister and G Stille (eds.), Springer-Verlag, Berlin, pp. 43-158, 1977.
Meyer DR and Sparber SB, Evidence of possible opiate dependence during the behavioral depressant action of a single dose of morphine, Life Sci 21: 1087-1094, 1977.
Nakamura S, Tepper J, Young S, Ling N and Groves P, Noradrenergic terminal excitability: effects of opioids, Neurosci Lett 30: 57-62, 1982.
Neal BS and Sparber SB, Mianserin attenuates naloxone-precipitated withdrawal signs in rats acutely or chronically dependent upon morphine, J Pharmacol Exp Ther 236: 157-165, 1986. Nestler EJ and Tallman JF, Chronic morphine treatment increases cyclic AMP-dependent protein kinase activity in rat locus coeruleus, Mol Pharmacol 33: 127-132, 1988.
Nestler EJ, Erdos JJ, Terwilliger RZ, Duman RS and Tallman JF, Regulation of G-proteins by chronic morphine treatment in the rat locus cocruleus, Brain Res 476: 230-239, 1989.
Nestler EJ, Hope BT and Widnell KL, Drug addiction: A model for the molecular basis of neural plasticity, Neuron 11: 995-1006, 1993.
Nishino K, Su YF, Wong CS, Watkins WD and Chang KJ, Dissociation of mu opioid tolerance from receptor down-regulation in rat spinal cord, J Pharmacol Exp Ther 253: 67-72, 1990. Pert CB and Snyder SH, Opiate receptor demonstration in nervous tissue, Science 179: 1011-1014, 1973.
Pert CB and Snyder SH, Opiate receptor binding - enhancement by opiate administration in vivo, Biochem Pharmacol 25: 847-853, 1976.
Pothos E, Rada P, Mark GP and Hoebel BG, Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment, Brain Res 566: 348-350, 1991.
Rasmussen K, Beitner-Johnson D, Krystal JH, Aghajanian GK and Nestler EJ, Opiate withdrawal and the rat locus coeruleus: behavioral, electrophysiological, and biochemical correlates, J Neurosci 10: 2308-2317, 1990.
Robles LI, Barrios M and Baeyens JM, ATP-sensitive K' channel openers inhibit morphine withdrawal, Eur J Pharmacol 251: 113-115, 1994.
Rogers NF and El-Fakahany EE, Morphine-induced opioid receptor down-regulation detected in intact adult rat brain cells, Eur J Pharmacol 124: 221-230, 1986.
Romandini S, Cervo L and Samanin R, Evidence that drugs increasing 5-hydroxytryptamine transmission block jumping but not wet-dog shakes in morphine-abstinent rats: a comparison with clonidine, J Pharm Pharmacol 36: 68-70, 1984.
Simon EJ, Hiller JM and Edelman I, Stereospecific binding of the potent narcotic analgesic ['H]Morphine to rat-brain homogenate, Proc Natl Acad Sci USA 70: 1947-1949, 1973.
Spanagel R, Herz A and Shippenberg TS, The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study, J Neurochem 55: 1734-1740, 1990. Tanganelli S, Antonelli T, Morari M, Bianchi C and Beani L, Glutamate antagonists prevent morphine withdrawal in mice and guinea pigs, Neurosci Lett 122: 270-272, 1991.
Tao P-L, Lee C-R, Law P-Y and Loh HH, The interaction of the mu-opioid receptor and G protein is altered after chronic morphine treatment in rats, Naunyn Schmiedeberg's Arch Pharmacol 348: 504-508, 1993.
Terenius L, Characteristics of the 'receptor' for narcotic analgesics in synaptic plasma membrane fraction from rat brain, Acta Pharmacol Toxicol 33: 377-384, 1973.
The RBI handbook of receptor classification, JW Kebanian and JL Neumeyer (eds.), Research Biochemical Inst, Natick, 1994.
Wang YY and Aghajanian GK, Excitation of locus coeruleus neurons by vasoactive intestinal peptide: role of cAMP and protein kinase A, J Neurosci 10: 3335-3343, 1990.
Way EL, Loh HH and Shen F, Simultaneous quantitative assessment of morphine tolerance and physical dependence, J Pharmacol Exp Ther 167: 1-8, 1969.
Wei E, Loh HH and Way EL, Quantitative aspects of precipitated abstinence in morphinedependent rats, J Pharmacol Exp Ther 184: 398-403, 1973a.
Wei E, Loh HH and Way EL, Brain sites of precipitated abstinence in morphine-dependent rats, J Pharmacol Exp Ther 185: 108-115, 1973b.
Wise RA and Bozarth MA, A psychomotor stimulant theory of addiction, Psychol Rev 94: 469492, 1987
Zito KA, Vicker G and Roberts DC, Disruption of cocaine and heroin self-administration following kainic acid lesions of the nucleus accumbens, Pharmacol Biochem Behav 23: 1029-1036, 1985.
Zweben JE and Payte JT, Methadone maintenance in the treatment of opioid dependence. A current perspective, West J Med 152: 588-599, 1990
| < Prev | Next > |
|---|












