4 Methodology
| Reports - Models of Good Practice in Drug Treatment |
Drug Abuse
4 Methodology
The methodology of this project can be divided into five consecutive phases.
Phase I: Definition and collection of European drug treatment modalities
Phase II: Methods for the preparation of an inventory of good practice
• Description of drug treatment interventions
• Identification of evidence of effectiveness of drug treatment interventions
• Data abstraction form
Phase III: Formal aspects of consensus process
Phase IV: Consensus building about drug treatment/interventions
Phase V: Disseminating of the results
4.1 Phase I: Definition and collection of treatment modalities in Europe
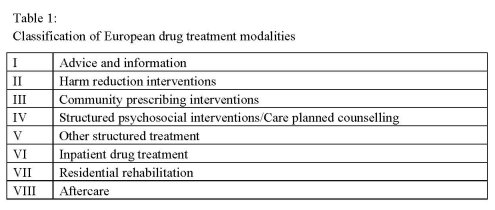
Phase I formed the base for the general methodology by specifying the methodological
approaches of the project. In a first step, given European drug treatment modalities from
different regions/countries were collected and predefined in terms of a general
classification of European drug treatment modalities (Table 1). Each identified
European drug treatment intervention was assigned to the respective classification,
which were taken as the basis for the formulation of draft treatment improvement
guidelines for drug treatment modalities in Europe.
4.2 Phase II: Preparations for an inventory of good practice in drug treatment
The second phase focused on the principle preparations for the inventory of European
drug treatment interventions.
Description of drug treatment interventions
On the basis of a detailed description of drug treatment interventions the structure of
the draft treatment improvement guidelines were defined, which included general (e.g.
objective, aim, context) and specific conditions (e.g. eligibility criteria, access to care)
of drug treatment interventions as well as aspects for their assessment (e.g. outcome
monitoring, process management, standards).
Identification of evidence of effectiveness of drug treatment interventions
The identification of evidence of effectiveness of drug treatment interventions was
carried out on two different ways. For the identification of international evidence a
comprehensive search of international electronic literature databases was carried out.
This strategy included also a search on internet based platforms of international
organisations in the field of drug research in the European Union, but also from outside
of Europe. A systematic search strategy (Annex #) based on combinations of index and
free text search terms was developed and adapted to the respective electronic literature
database. Due to the wide range of research literature on the efficacy and effectiveness
of drug treatment interventions, the analysis was limited to the findings of the latest
experiments in the form of “Randomized Controlled (Clinical) Trials” (RCTs), metaanalyses/
systematic reviews including RCTs or at least clinical trials. From a
methodological point of view, RCTs have the highest evidential value in terms of
efficacy, because they are less susceptible to methodological biases. Therefore, a
comprehensive literature review was carried out, including a systematic search strategy
to identify all relevant randomised controlled trials and clinical trials (see search
strategy). In the absence of RCTs this the strategy also refers to less rigorously designed
studies (such as observational studies), case series and unsystematic reviews. In addition
to general limitations (heterogeneity of the assessment of outcomes, widely varying
approaches with regard to duration, design and treatment objectives etc.), the inclusion
of less rigorously designed studies may lead to limitations. The searched relevant
international electronic databases were MEDLINE, EMBASE, DARE, Central register
of the Cochrane library, Database of Health Technology Assessment (HTA) and
PsychInfo (Table 2).

The specification and sensitivity of the search strategy was refined through literature
scoping. Several general limits and selection criteria were set to avoid analysis iterations
(Table 3).

Additionally, available on-line and electronic journals in the field of addiction were also
searched for further relevant publications. All publications identified through literature
search in international databases were assessed in a first analysis of the abstracts and the
full text was ordered of the literature considered relevant. The results of the search on
the efficacy and effectiveness of drug treatment interventions in international databases
are placed in front of the results part.
Besides this general search of international electronic literature databases, an adapted
search strategy was developed for each European region/country to identify “European
national drug treatment interventions”, which are only available in national databases
and national journals, mainly written in the respective national language. All associated
partners carried out this specific search for their respective European region.
Besides the collection of data and literature about the evidence and effectiveness of
these interventions through searching in national databases, contacts to national experts
in the area of drug treatment were established to gather further information and
literature. Regional reviews were screened for further literature by using the method of
reference tracking. This method was also applied for the search for evidence in the
national reports of the REITOX Network of the European Monitoring Centre for Drugs
and Drug Addiction (EMCDDA) and project reports of medical and non-medical
research bodies working in field of drug treatment.
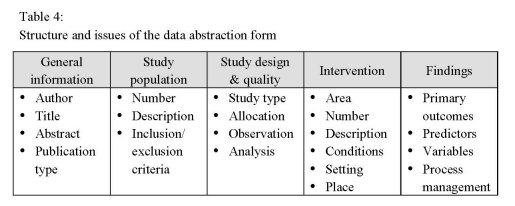
Data abstraction form
In order to establish a common platform for the exchange of information about national
European drug treatment interventions, the database programme FileMaker was used to
prepare a data abstraction form for detailed description and further analysis. On the
basis of a specific manual, including explanations, definitions and help, relevant
information from identified national publications were extracted and entered in the data
form (Table 4). If necessary, parts or the whole publication were translated into English
language.
In order to provide all gathered information about the defined treatment areas for all
project partners, each get free online access to the database. On the basis of this
common platform, each project partner obtained the relevant information for the
development of his/her respective draft treatment improvement guideline. Besides this,
each partner received a literature database including the results of the general systematic
search strategy in international databases.
4.3 Phase III: Development of consensus process
For the development of consensus a nominal group technique (Delphi method) was
applied. The Delphi method is a formal consensus process providing the possibility to
discuss and resolve open questions. The Delphi method is an interactive and systematic
method, which provides an exchange platform for independent experts. In one or more
rounds the experts give their opinion to a specific question. The process stops normally
after a pre-defined stop criterion (e.g. number of rounds, achievement of consensus, and
stability of results). Adapted to this project several experts were selected to discuss the
recommendations of the draft and final version of the treatment improvement
guidelines.
4.4 Phase IV: Consensus building about drug treatment/interventions
This phase focussed on the achieved consensus of experts in the different areas of drug
treatment/interventions under consideration of the different groups of professionals,
commissioners and providers of drug treatment.
To realise the formal consensus process via adapted Delphi-method, a consensus
conference in Hamburg (3 days) was organised. Before the consensus conference the
draft treatment improvement guidelines were disseminated to the involved project
partners including a short questionnaire with closed and open questions, which should
highlight potential barriers to consensus building. On the basis of the reviewed draft
treatment improvement guidelines open questions were discussed and resolved.
4.5 Phase V: Dissemination of the results
For the dissemination of the project results a strategy was developed, taking into
account the different project deliverables as well as the relevant different target groups
for dissemination.
Projects results for dissemination are:
• Fact sheets of good practice treatment improvement guidelines
• Full version of good practice treatment improvement guidelines
• Executive summary of the final report
• Final report
Target groups for dissemination are:
• Organisations and service provider
• National and European networks
• National authorities and policy decision maker
• European stakeholder
Table 5 shows relevant target groups with regard to the respective project deliverable.
From each treatment improvement guideline fact sheets were developed for short
communication, wide spread and awareness rising. For a deeper understanding and for
more information regarding specific drug treatment modalities, full versions of the
treatment improvement guidelines will be provided to European drug treatment
provider. The executive summary of the final report provides the opportunity to
disseminate a short communication form to European stakeholder in the field of drug
treatment. The final report will be disseminated to relevant networks, services and
organisations as well as to public health authorities and drug treatment commissioners
in the European Member States.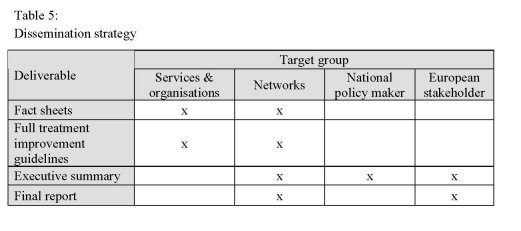
| < Prev | Next > |
|---|












