THE NATURAL AND SYNTHETIC MATERIAL
| Reports - Marihuana and Health |
Drug Abuse
THE NATURAL AND SYNTHETIC MATERIAL
As illicitly sold, what is called marihuana in the United States may vary from carefully prepared plant material of high potency to psycho-actively inert materials masquerading as marihuana. Such adulterants as catnip, oregano and tea are sometimes part. of the mixture in order to increase the profit of the seller. The less sophisticated the buyer the more likely he is to obtain inferior or substitute material. Because of the important role that psychological factors play in the effect of psychoactive drugs, users may obt ain a subjective high even though the substance used is actually inert or very nearly so. Thus it is important to recognize at the outset that marihuana and related materials encompass an unusually wide range of substances with highly variable psychoactive potential. At least part of the current emotionally charged debate over this group of substances is the result of a failure to adequately specify the material both by dosage and level of psycho-activity, usually expressed in terms of the percentage of Delta 9—THC (the presumed principal psychoactive ingredient) contained in a given sample. Most early research suffers from the serious deficiency that it is impossible to be certain what dosage and potency of material were used.
PLANT MATERIAL
What is commonly called marihuana in North America consists of a mixture of crushed leaves, flowers and often twigs of the Indian hemp plant. This herbaceous annual readily grows in temperate and tropical climates in many parts of the world including the United States, and can reach 15-20 feet in heir (4). Although there are many varieties, it is now generally agree( that they all belong to a single species, Cannabis Sativa, which exhibit variations because of genetic plasticity and different environmental conditions (13) . Cannabis is dioecious, i.e., it has separate male (staminate) and female (pistillate) plants. The male plants are taller and short lived, usually dying after their pollen is shed. The female plants are bushier, pollinate and survive until killed by frost or their seeds are fully mature. Both types are indistinguishable until the flower buds are well developed. Male flower clusters usually have little foliage and are borne in leaf axils as loosely arranged clusters. The female clusters are more densely packed. Complete descriptions of the morphology and botanical characteristics of Cannabis sativa have been published (12, 3, 14). The flowering tops of the female plant secrete a clear, varnish-like resin called "hashish" in the West and "charas" in India. They contain the most concentrated psychoactive material.
Cannabis preparations containing plant materials of varying potency include bhang and ganga (India), maconha (Brazil), kif and dagga (Africa). The two Cannabis preparations most commonly used m the United States are native or imported marihuana and imported hashish. It is well known that marihuana froniclifferent areas differs in potency and that drug users prefer certain sources for their higher. potency. Preferred types are : "Panama Red, Acapulco Gold, Texas Black and Vietnam Red." Using analytical methods such as gas chromatography, it is now possible to relate the differences in potency to the difference's in the chemical composition of marihuana from, these various sources. It has been shown that the amount and ratio of the components in marihuana are a function of innate botanical (genetic) factors and conditions of growth.- The mode of preparation of the crude plant material and the conditions of its storage, such as exposure to heat and elapsed time since harvesting are also important.
Cannabis has spread throughout the United .States along the major rivers and there is a correlation between Cannabis distribution and alluvial stream deposits in areas of the plain states where intermittent flooding occurs (6).
'Contrary to prior beliefs, recent investigations have shown that both the male and female plants contain psychoactive material (16). The various parts of the plants differ, however, in the percentage of active principles they contain, with the flowering tops, bracts and leaves having the highest percentage of tetrahydrocannabinols, and the stems, seeds and roots the least. The mode of preparation of marihuana becomes, therefore, quite important. A "carefully manicured" sample containing mostly the upper parts of the plant is typically more potent than one containing a higher proportion of stems and leaves. The resin itself contains five to ten times more psychoactive ingredient than the leaves.
It is now believed that there are two genotypes of marihuana—the drug type with a high percentage of tetrahydrocannabinol (1-5%) and the fiber type with a high percentage of cannabidiol (17). Analysis of wild Midwestern marihuana (Iowa, Indiana) has shown that the plants contained predominantly cannalsidiol (CBD) with only small amounts of THC. Cyclic peaking of cannabidiol occurs during the growing season, with the THC content inversely proportional to the CBD content. THC content is usually low on the same day that the cannabidiol is high and vice versa (11). This suggests that cannabidiol may be a precursor of THC in the plant as proposed by Mechoulam (8).
CHEMISTRY OF MARIHUANA
The variation in potency between different sources which has hampered research on marihuana, could not be explained until the mid 1960's when the structure of the active components of marihuana was finally elucidated (5). Up to that time, the situation for marihuana contrasted sharply with that for other drugs of abuse, such as morphine and cocaine. These drugs, also originating from natural sources and used for illicit purposes, were well known chemical entities and research on their effects could be easily duplicated.
Prior to 1964, the biologists and clinicians studying marihuana believed that the chemistry of marihuana components had been elucidated by early studies of Adams and Todd in the 1940's. Numerous “cannithinoids" were known to be present in the resin and the plant but the structure of only one, carmabinol, had been fully elucidated (1, 15).
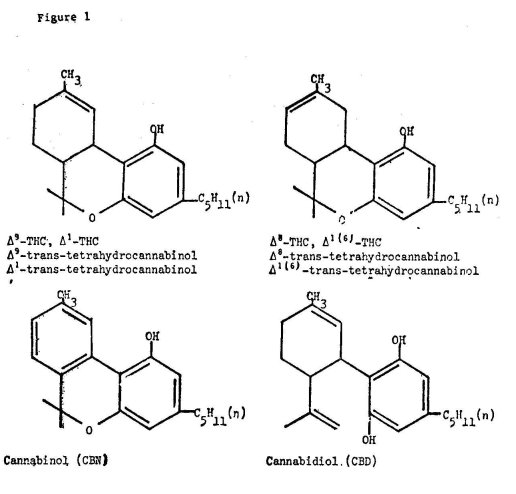
The term "cannabinoids" as generally used includes all the C21 compounds typical of and present in Cannabis sativa, their carboxylic acid analogues and their transformation products. In the last few years, intensive investigations have . clarified considerably the. rather complex chemistry of marihuana. Most natural cannabinoids in the plant have now been isolated and purified, their structures elucidated and analytical methods for their detection and quantification developed. In the period from 1963 to 1968, the true structure of the tetrahydrocannabmols was clarified and it was shown that a double bond in the synthetic tetrahydrocannabinol structure described in 1940 was in a different position in the natural tetrahydrocannabinol extracted from the plant (9). At present, four major cannabinoids have been found in the plant: the two isomers: ( — )-trans-Delta-9 and Delta-8- tetrahydrocannabinols (Delta-9-THC and Delta-8-THC), cannabidiol (CBD) and cannabinol (CBN). Their formulas are shown in Figure 1. The major tetrahydrocannabinol believed to be responsible for the psychoactive properties of marihuana is the Delta-9-THC. Other minor cannabinoids present in marihuana include cannabigerol, cannabicyclol, cannabichromene and cannabidivarin. The structure of these compounds was determined by extensive use of modern physical techniques such as nuclear magnetic resonance spectroscopy, mass spectrometry, and, most recently, proton magnetic resonance (2).
The nomenclature of the cannabinoids is rather confusing. As many as four numbering systems have been proposed and two different systems are actually used with about equal frequency. In one, the formal chemical rules for numbering pyran-type compounds are used because the tetrahydrocannabinols are substituted dibenzopyrans. In the other, the cannabinoids are regarded as substituted monoterpinoids and numbered accordingly. Thus, the major constituent of marihuana is referred to either as Delta-9-THC or Delta-l-THC, and its insomer as Delta-9-THC or Delta-1 (6) -THC.
In addition to the neutral cannabinoids described above, cannabinoidic acids have been isolated (7). They differ from the neutral cannabinoids only by the presence of an additional carbolyxic group in the molecule. Depending on the position of the carbolyxic group in the benzene ring, two THC acids have been isolated: A and B. Rapidly upon heating, or slowly after storage, the acids can be decarboxylated to the corresponding neutral cannabinoids. In order to quantify the percentage of acids present in the plant by gas chromatography, a method was developed (PH-43-68-1338) which prevents decarboxylation of the acids in the gas chromatograph by transforming them to trimethylsylyl derivatives. Routine analysis of marihuana samples performed by NIMH (HSM-42-70-17) has shown that most of the THC (70-90%) contained in the fresh marihuana plant was in the form of acids. Investigation of the amount of cannabinoidic acids at different times and in different parts of the plant has shown that it is higher in the parts of rapid growth and especially concentrated at the bractlet during the period when the seeds are at the peak of ripening.
SYNTHETIC MATERIAL
Once Delta-9-THC became known as the principal psychoactive component of marihuana, a number of synthetic methods for producing it were proposed since extraction of THC from the natural plant material is both difficult and low in yield. However, they usually involved many steps and were long, tedious and expensive. Late in 1967, Petrzilka, in Switzerland published the first elegant and simple synthesis of Delta-8 and Delta-9 by condensation of trans-p-menthadiene (2,8) -1-o1 with olivetol. (10) This method was further developed in the United States under NIMH contract (P11-43-68-1339) for larger scale production and has made possible the production of sufficient quantities of these materials to satisfy research needs. Methods of synthesis and analysis are being constantly improved and it is now possible to get 95% pure Delta-9-THC free of non-volatile material by rechromatography and redistillation. This better product contains fewer impurities than before and should prove to be more stable. The lack of stability of Delta-9-THC when exposed to air, light or increases in temperature has been one of the problems connected with the synthetic material. These problems have not been encountered with Delta-8-THC since it is more stable and could be produced from the beginning in relatively pure form (98%). Limited amounts of the other cannabinoids such as cannabinol, cannabidiol and the 11-hydroxyDelta-9-THO metabolite have also been synthesized and made available for research (NIMII contract P11-43-68-1452), mostly for use as analytical standards. Unfortunately, except for the cannabidiol which comes in a crystalline form, the other components of marihuana are oily, viscous materials both difficult to handle and to convert to convenient forms for administration. Ways are now being studied to make intravenous, oral and aerosol preparations which can be used in clinical studies (NIMII contract HSM-42-70-145). In view of the importance of the acids, they are also being prepared. As for the other marihuana components of research interest, depending on yield and available methods of synthesis, decisions will have to be made whether they should be extracted from the plant or made synthetically.
EXTRACT
A material called marihuana extract distillate (M.E.D.) was prepared under contract by the National Institute of Mental Health (NIMI1) in 1960 and contained 17% Delta-0-THC. Unfortunately, this extract was made from seized marihuana (before the NI14111 started to grow its own product) and contained a large percentage of fatty acids not usually present in the plant. Extracts are now being produced under contract which contain as much as possible of the materials present in the fresh plant. The solvent used is usually petroleum ether and extraction is made at low temperature. The preparation of a standard marihuana extract and its testing is necessary as long as there is not complete agreement that Delta-9-THC, is the only compound
responsible for marihuana's behavioral and psychoactive effects. Other extracts have also been prepared by various investigators.
IMPURITIES OP MARIHUANA
Although marihuana and the active ingredients of the hemp plant are the focus of this report., it must be recognized that other ingredients are sometimes found in the material that is smoked or ingested. Users are exposed to a wide variety of additives, diluents and contaminants, since marihuana is available only through illicit channels and systematic quality control is non-existent.
The frequency of mixtures containing other psychoactive materials, whether of natural or synthetic origin, is not known. Nor is there any reliable information about the effect of these contaminants when present.. It is clear, however, that an almost limitless number of compounds are available as possible contaminants ranging from deliberately added adulterants to inadvertent pollution by herbicidal action.
At the present time there are no means by -which users can readily determine whether or not contaminants are present in marihuana. la direct contracts to drugs which have been diverted from legitimate channels with assurance of at least initial quality control, marihuana is always dependent upon the vagaries of the illicit distribution system for whatever purity it has.
Two reports give an indication of the magnitude of this aspect of the marihuana problem. Marshman and Gibbins present data from. Ontario for 222 samples collected during the first eight months of 1969 (6.a). The channels threnigh...whichthese' Samples were collected are not described. Of the 222 samples, it Was claimed..that 13% were hashish and 11% were marihuana,. Of the total number of 222 samples, the composition was determined on 197 samples with 61.9% containing the drug that was alleged to be present. Of those samples alleged to be hashish, 100% were hashish.; Of those alleged to be marihuana, 67% contained marihuana.: .• •
The report states : "In regard to 4he,36 samples alleged to be marihuana, with a high cannabinoid content, "good grass," its it,would be ermed On the street, some were marih,uana cut with other suNtances and some contained no marihuana at all. Some of it appeared literally to be grass-lawn clippings; some of it looked like hay and smelled like hay. Our figure of 61 per cent for samples that 'contained marihuana' includes all the samples that contained any marihuana at all. It is clear that a sizeable portion of what is sold and smoked is not marihuana but other substances, sometimes of unknown origin."
A report. by the Bureau of Narcotics and Dangerous Drugs' Laboratory Operations Division states that during the fourth quarter of fiscal year 1970, a total of 1645 exhibits of suspected marihuana were analyzed (3a). Qualitative analysis showed negative results for 12% of the total or 191 exhibits. Even with use of a large number of specimens, the false positive claim rate fluctuated : 14% for the first quarter, 16% for the second quarter and 7% for the third quarter.
REFERENCES-PART III-MATERIAL
1. Adams, R. et al. Structure of cannabinol. III. Synthesis of cannabinol. Journal of the American Chemical Society, 62, 2204-2207, 1940.
2. Archer, R. A. et al. Structural studies of cannabinoids. A theoretical and proton magnetic resonance analysis. Journal of the American Chemical Society, 92 (17), 5200-5206, 1970.
3. Bouquet, J. R. Cannabis. Bulletin of Narcotics, 2:14-30, 1950.
3a. Marshman, J. A. & Gibbons, R. J. The credibility gap in the illicit drug market. Addiction 16:4, Winter, 1969.
4. Farnsworth, N. R. Pharmacognosy and chemistry of Cannabis sativa. Journal of the American Pharmaceutical Association, NS 9 (8) : 410-414, 1969.
5. Craoni, Y. & Mechoularn, R. Isolation, structure and partial synthesis of an active component of hashish. Journal of the American Chemical Society, 86, 1646-1647. 1964.
6. Haney, A. & Bazzaz, F. A. Some ecological implications of the distribution of hemp ( Cannabis sativa L.) in the United States of America. In: The Botany and Chemistry of Cannabis. Joyce, C. R. B. and Curry, S. H., Eds. J & A Churchill, London, 1970, pp. 39-48.
6a. Bureau of Narcotics and Dangerous Drugs, Laboratory Operations Division. Washington, D.C., October 1970. Private communication.
7. Mechoulam, R. et al. A new tetrahydrocannabinolic acid. Tetrahedron Letters, 28, 2339-2341, 1969.
8. Mechoulam, R. Marihuana chemistry. Science, 168, 1159-1166, 1970.
9. Mechoulam, R. & Gaord, Y. The absolute configuration of Delta-1-tetrahydroca nnabinol, the major active constituent of hashish. Tetrahedron Letters, 12, 1109-1111, 1967.
10. Petrzilka, T. & Sikemeier, C. Components of hashish. IL Synthesis of (–)– Delta."-3,4-trans-tetrahydrocannabinol and (+) —Delta61-3,4-trans-tetrahydrocannabinol. Helvetica Chim. Acta, 50, 1416-1419, 1967.
11. Phillips, R. et al. Seasonal variation in cannabinolic content of Indiana marihuana. Journal of Forensic Science, 15 (2 ) 191-200, 1970.
12. Ram, H. Y. & Nath, R. The morphology and embryology of Cannabis sativa Linn. Phytomorphology, 14: 414-429, 1964.
13. Shultes, R. E. Random thoughts and queries on the botany of Cannabis. In: The Botany and Chemistry of Cannabis. Joyce, C. R. B. & Curry. S. H., Eds. J & A Churchill, London, 1970, pp. 11-38.
14. Steam, W. T. The Cannabis plant botanical characteristics. In: The Botany and Chemistry of Cannabis. Joyce, C. R. B. & Curry, S. H., Eds. J & A Churchill, London, 1970, pp. 1-10.
15. Todd, A. R. The chemistry of hashish. Scientific Journal of the Royal College of Science, 12, 37-45, 1942.
16. Valle, J. R., Lapa, A. J. & Barros, G. G. Pharmacological activity of Cannabis according to the sex of the plant. Journal of Pharmacy & Pharmacology, 20: 798-799, 1968.
17. Waller, C. W. Supplies for the marihuana program. (Report to the Committee on Problems of Drug Dependence), Feb. 36, 1970.
| < Prev | Next > |
|---|












