Chapter 9 Harm reduction policies for tobacco
| Reports - EMCDDA Harm Reduction |
Drug Abuse
Chapter 9 Harm reduction policies for tobacco
Coral Gartner, Wayne Hall and Ann McNeill
Abstract
Tobacco smoking is the leading cause of preventable premature mortality and disability in
European and other developed countries. This chapter first reviews strategies that (1) aim to
reduce harm to non-smokers (public smoking bans and reduced ignition propensity
cigarettes) and (2) aim to reduce harm to the smoker who is unable or unwilling to quit
nicotine use, namely, regulating the harmfulness of cigarettes, and encouraging smokers to
switch to less harmful nicotine products. The putative tobacco harm reduction products
discussed include: modified tobacco cigarettes and cigarette-like devices, smokeless tobacco
products and pharmaceutical nicotine products. The evidence for the harm reduction
potential of each of these is discussed, as are adverse public health outcomes that may
potentially arise from their promotion. The chapter concludes with a description of the most
promising options for promoting tobacco harm reduction.
Keywords: smokeless tobacco, snus, reduced ignition propensity cigarettes, smoking bans,
potential reduced exposure products, pharmaceutical nicotine.
Introduction
Tobacco can be smoked as cigarettes, in a pipe, or as cigars or used via non-smoked
products such as chewing tobacco or oral and nasal snuff. Nicotine is the primary substance
responsible for tobacco dependence but the majority of harm caused by tobacco use is not
from nicotine but from the by-products of smoked tobacco (e.g. fine particulates, carcinogens,
and noxious gases including carbon monoxide). Cigarettes are the most addictive and
hazardous tobacco product, because cigarette smoke is readily drawn deep into the lungs
where it is rapidly absorbed into the bloodstream and from which nicotine quickly reaches
the brain (Benowitz, 2008).
In Europe, as in many regions of the world, the cigarette has become the dominant form of
tobacco use over the past century (Berridge, 2007). The rise in the popularity of the cigarette
was followed with a lag of several decades by increases in tobacco-caused diseases
including cancers, pulmonary and cardiovascular diseases. By mid century tobacco smoking
had become the leading cause of preventable premature mortality and disability in Europe
and other developed countries. Cigarette smoking is currently responsible for around
730 000 deaths in the European Union (EU) each year (including 80 000 from passive
smoking) (ASPECT Consortium, 2004).
Smoking prevalence has declined in most western European countries over the past 40 years,
but prevalence remains high in many eastern European countries (ASPECT Consortium,
2004; WHO Regional Office for Europe, 2007). The disparities in smoking prevalence across
Europe largely reflect differences in the intensity with which tobacco control policies have
been implemented, such as increasing cigarette taxes, banning cigarette advertising, public
mass media anti-smoking campaigns and restricting smoking in indoor public spaces
(ASPECT Consortium, 2004; WHO Regional Office for Europe, 2003; WHO Regional Office
for Europe, 2007).
Policies that encourage existing smokers to quit and discourage non-smokers from starting
remain the most effective ways of reducing tobacco-related harm (World Bank, 2003).
Nonetheless, even in countries that have most rigorously enforced these types of policies
(Australia, the United States, Canada, the United Kingdom and Sweden), none have reduced
overall smoking prevalence below one in six adults. Plausible projections show that more
than 10 % of adults will be smoking in another 20 years if current rates of cessation and
initiation continue (Gartner et al., 2009; Kemm, 2003; Mendez et al., 1998).
The persistence of smoking in a substantial minority of adults has prompted some to
advocate tobacco harm reduction (THR) policies as an addition to conventional strategies
that promote abstinence from tobacco. Harm reduction policies are generally those that
‘attempt to prevent problems by targeting risky contexts or patterns of use, or by moderating
the relation between use and problem outcomes, without necessarily affecting overall rates of
use’ (Toumbourou et al., 2007, pp. 1398–9). In the case of THR, this approach involves
attempting to reduce the harmfulness of tobacco use without necessarily advocating cessation
or abstinence, typically by advocating the use of much less harmful forms of tobacco or
nicotine use.
Policies that reduce the harm to others
Public smoking bans
Non-smokers who are exposed to second-hand smoke (the emissions from the end of lit
cigarettes and the exhaled smoke from a smoker) are at increased risk of many of the same
diseases that affect smokers (US Department of Health and Human Services, 2006). Workers
in smoky environments, such as bar staff, are particularly at risk due to their regular and
prolonged exposure. Legislated bans on smoking in enclosed public spaces such as office
buildings, restaurants, cafes, bars and clubs provide protection of employees and patrons
and are the most widespread and non-controversial tobacco harm reduction policy. Research
has shown that public smoking bans in countries like the United States and Australia have
been effective in reducing exposure to second-hand smoke in these previously smoky
environments (Hopkins et al., 2001). There is also evidence that these policies can provide
immediate population health improvements, such as a reduction in the number of
hospitalisations for acute coronary events (Pell et al., 2008).
A number of European countries have recently introduced indoor public smoking bans (for
example, Republic of Ireland, United Kingdom), but many countries still do not have
comprehensive smoking bans (Joossens and Raw, 2007). To be effective at reducing the
exposure of non-smokers, these bans need to cover all enclosed areas and should also
extend to outdoor areas that are serviced by waiting staff. Smoking bans also have the
added benefit of increasing cessation in the smoking population by reducing the
opportunities to smoke and contributing to the de-normalisation of smoking (Fichtenberg
and Glantz, 2002).
Reduced ignition propensity (RIP) cigarettes
Fires started by cigarettes cause substantial damage to property and loss of life. Internal
tobacco industry documents show that the industry knew how to reduce the ignition
propensity of cigarettes many years ago (Gunja et al., 2002) by reducing tobacco density,
paper porosity and cigarette circumference, eliminating burn additives and by increasing
the length of filters (Chapman and Balmain, 2004). Legislation requiring cigarettes to meet
RIP performance standards has now been implemented in 22 US states and Canada
(Arnott and Berteletti, 2008). In 2007, the EU Member States endorsed plans to develop a
mandatory standard to reduce the ignition propensity of cigarettes sold in the EU (Arnott
and Berteletti, 2008; Commission of the European Communities, 2008). An evaluation of
New York’s RIP standard (implemented in 2004), showed that it substantially reduced the
ignition propensity of cigarettes sold in that state, largely via ‘paper banding’, without
increasing the toxicity of the emissions (Alpert et al., 2005). There is as yet no evidence that
the introduction of RIP standards has reduced cigarette-related fires. Nevertheless,
implementation of a RIP performance standard in Europe would not be costly to the public,
would have very little risk of producing adverse outcomes and could reduce the number of
fires caused by discarded cigarettes.
Policies that reduce harm to the smoker
The main putative tobacco harm reduction products in order of decreasing relative
harmfulness are modified tobacco cigarettes and cigarette-like devices, smokeless tobacco
(SLT) products and pharmaceutical nicotine (PN) products (Stratton et al., 2001).
Modified tobacco cigarettes and cigarette-like devices
Regulating the harmfulness of cigarette emissions
The tobacco industry began developing a ‘safer’ cigarette in response to the emerging
evidence of the harm from cigarette smoking in the 1950s (Glantz et al., 1996). The first
example was the filtered cigarette, followed by so-called light, low-tar and low-nicotine
cigarettes in the 1980s (Stratton et al., 2001). These cigarette modifications, which consisted
of the addition of tiny ventilation holes in the side of the filter to dilute the smoke with air
drawn in through these holes, were popular with smokers; however, they did not reduce the
health risks of smoking as smokers compensated by drawing harder on the cigarette,
covering the filter ventilation holes and smoking the cigarettes down to a shorter butt length.
Research later revealed that the cigarette manufacturers knew these were not genuine
reduced harm products, but marketed them to reassure health-conscious smokers and
discourage quitting (Glantz et al., 1996).
The World Health Organization’s Study Group of Tobacco Product Regulation (TobReg)
advocates mandatory maximum permissible levels of key toxicants in mainstream cigarette
smoke (Burns et al., 2008) and the tobacco industry has developed and marketed cigarettes
made with low nitrosamine tobacco and carbon filters, both of which are claimed to expose
smokers to fewer toxins than regular cigarettes (Hatsukami et al., 2004; Rees et al., 2008). A
major problem with this approach is that reductions in some toxins are often achieved by
increasing others (King et al., 2007). Given that tobacco smoke contains more than 4 000
different chemicals, it will be difficult to achieve a substantial reduction in overall harmfulness
(Stratton et al., 2001). Futhermore, there is no evidence that reducing or removing known
toxins in cigarettes will produce observable reductions in smoking-related lung cancer
(Pankow et al., 2007), yet publicity around mandating these changes may give consumers
the impression that they do significantly reduce harm. Monitoring and enforcing a cigarette
emissions standard will also require substantial laboratory and regulatory resources that may
arguably be better used in other ways.
Cigarette-like devices
The tobacco industry has also marketed cigarette-like devices that aim to minimise tars
and maximise nicotine by heating tobacco to produce an aerosol or vapour rather than
smoke (for example, Eclipse, Premier, Accord and Heatbar) (Shiffman et al., 2002a;
Stratton et al., 2001). Some of these products reduce emissions of one or more key toxins,
but some studies report higher emissions of others (Breland et al., 2002; Breland et al.,
2006; Fagerström et al., 2000; Stratton et al., 2001). Given the long latency of many
tobacco-related diseases, it will take several decades before we know whether these
products substantially reduce tobacco-related mortality and morbidity. Given these
difficulties, we should arguably abandon attempts to reduce the harmfulness of cigarette
emissions by modifying cigarettes or producing cigarette-like tobacco products in favour
of harm reduction using non-smoked forms of tobacco and clean nicotine products
(Stratton et al., 2001).
Smokeless tobacco (SLT) products
SLT products present greater opportunity for THR than smoked tobacco because there is
no combustion/vaporisation and therefore no risk of respiratory disease, fire or passive
smoking. SLT products include traditional chewing tobacco and snuff, and new products
such as compressed tobacco lozenges, tobacco chewing gum and dissolvable strips
(Hatsukami et al., 2007; Stepanov et al., 2006). Most policy attention has focused on a
form of moist oral snuff used in Sweden, known as snus (see box on p. 262). It has much
lower levels of tobacco-specific nitrosamines than snuffs marketed in the United States and
elsewhere because it is produced by pasteurisation rather than fermentation (Hoffmann et
al., 1995; Österdahl et al., 2004; Ramström, 2000). Levels of nitrosamines in Swedish snus
have decreased over the past 20 or so years in response to the development of an
industry standard (Hatsukami et al., 2007; Österdahl et al., 2004). The development of
portion snus in the 1970s (tea-bag-like sachets of snus) has produced a more user-friendly
version that has increased prevalence of snus use among Swedish men. The fact that until
recently snus was taxed at a much lower rate than cigarettes may also have contributed
to its increased popularity. Increased snus use by Swedish men has been accompanied by
decreased cigarette smoking and tobacco-related disease mortality (Foulds et al., 2003;
Ramström, 2003).
A major barrier to the adoption of this form of harm reduction is the ban on the sale of the
least harmful smokeless tobacco products in many countries. In Australia and New
Zealand, for example, oral snuff and chewing tobacco products cannot be sold
(Commonwealth of Australia, 1974; Parliament of New Zealand, 1990). With the exception
of Sweden, the same is true in all EU Member States, where the sale of these tobacco
products is prohibited, although chewing tobacco and nasal snuff can be sold (European
Court of Justice, 2004).
Pharmaceutical nicotine (PN)
PN products in the form of gum, patches, inhalers and sprays have been available for
many years. A new PN product under development is an oral nicotine pouch that mimics
portion snus (Fagerström and Jiménez-Ruiz, 2008). PN is generally a safe (except perhaps
in pregnancy), modestly effective and cost-effective way to help smokers to quit (Bertram et
al., 2007; Stead et al., 2008), or, potentially, also as a long-term alternative to cigarette
smoking (Warner et al., 1997). These products have minimal risk of abuse, in part because
of their design. The long-term use of PN appears to be safe, as no treated morbidity or
mortality was observed in five years of follow-up of nicotine gum users (Murray et al.,
1996). Long-term use of PN in ex-smokers may also help prevent relapse to smoking (Hajek
et al., 2007; Medioni et al., 2005).
The major disadvantages of PN are that, like other smoking cessation aids (bupropion,
varenicline), most smokers who use it do not succeed in quitting (Nides, 2008; Shiffman et
al., 2002b), and it has not been taken up by smokers as an alternative to smoking despite
its wide availability in many developed countries. This seems to be because these products
have been engineered for smoking cessation, with the aim of minimising their abuse by
delivering a lower nicotine dose at a slower speed to cigarettes. They are also not
marketed as long-term alternatives to tobacco smoking. For these products to gain
popularity, PN regulation would need to be relaxed to allow these products to be made
more attractive to inveterate smokers.
Recreational nicotine products
The marketing of the ‘e-cigarette’, a device that looks like a standard tobacco cigarette but
contains only nicotine in a carrier vapour, is a recent attempt to commercialise a
recreational nicotine product. Its similarity to cigarettes has led most tobacco control
advocates to refer to it as a cigarette-like device. The e-cigarette produces a propylene
glycol vapour and has a glowing red tip to simulate a lit cigarette. The manufacturers have
not marketed it as a smoking cessation aid and this has created regulatory barriers in
some countries (for example, Australia and New Zealand) (National Drugs and Poisons
Scheduling Committee, 2009; New Zealand Public Health Directorate, 2006). Some EU
Member States have defined e-cigarettes as medical devices and require them to obtain a
Confirmatory European (CE) mark before sale (e.g. Denmark, Austria) (Danish Medicines
Agency, 2009; European Commission Health and Consumer Protection Directorate-
General, 2008). A safety assessment of one brand of e-cigarette funded by the
manufacturer suggests the product may be relatively safe (Laugesen, 2008; Laugesen et
al., 2008), but there are no data on the patterns of use in smokers or uptake by nonsmokers
in countries where these products are sold, and there are no safety studies by
groups that are independent of the industry.
There are claims in the popular media in the United Kingdom that the e-cigarette is being
used in response to smoking bans in pubs and clubs (Sikora, 2007). Critics of the
e-cigarette also argue that it maintains a visible smoking-like behaviour that may
undermine the de-normalisation of smoking produced by public smoking bans (Chapman
and Freeman, 2008). The substantial cost of the device and its replacement cartridges, the
gimmicky nature of the smoke and glowing tip, and the regulatory hurdles in most
countries will probably limit its use for THR (Arendt, 2008). However, more data is needed
on whether smokers find these devices an acceptable substitute for smoking regular
cigarettes.
The e-cigarette illustrates the inadequacy of current regulatory structures. Claims about
aiding cessation would result in the e-cigarette being classified as a medicine and would
require safety, quality and efficacy data before being marketed. If no such claims are made,
the e-cigarette is likely to be regulated like tobacco cigarettes, and would then be subject to
all the regulations that apply to tobacco products. Neither set of regulations are appropriate
for e-cigarettes, the relative harmfulness of which is likely to fall somewhere between tobacco
cigarettes and PN.
Will tobacco harm reduction products reduce harm to users?
There is no evidence that modified smoked tobacco products and cigarette-like devices
substantially reduce harm. Experience with ‘light’ cigarettes also provides strong reasons
for not allowing them to be promoted as THR products (Stratton et al., 2001; Warner,
2001). ‘Light’ cigarettes failed to reduce harm in smokers due to compensatory changes in
the way they were smoked, such as inhaling more deeply, smoking a greater number of
cigarettes and more of each cigarette, and blocking ventilation holes designed to dilute
smoke exposure (Stratton et al., 2001). The mistaken image of a less harmful cigarette also
provided reassurance to health-concerned smokers, which discouraged quitting. Similar
compensatory changes, and/or ‘risk swapping’ by decreasing some toxins whilst
increasing others, and false reassurance of safety, are likely to limit any benefits from THR
products that involve the combustion or vaporisation of tobacco (e.g. Gray, 2004; Pierce,
2002; Stratton et al., 2001).
This argument does not apply to THR using PN and low nitrosamine SLT (LNSLT). The safety
of PN is well established in the short to medium term with users having been followed for
up to five years (Murray et al., 1996). PN may carry some residual health risks, such as an
increased risk of cardiovascular disease arising from chronic nicotine intake, and adverse
foetal outcomes if used in pregnancy, but these effects are small by comparison with those
of cigarette smoking (Benowitz, 2000). Literature reviews of the health effects of SLT
(Broadstock, 2007; Royal College of Physicians, 2007; SCENIHR, 2008) have concluded
that some forms of SLT such as Swedish snus, which is low in nitrosamines, are significantly
less harmful than smoking cigarettes. SLT use is not associated with respiratory diseases,
including lung cancer and chronic obstructive pulmonary disease (COPD), but some
potential health risks remain, namely oral and pancreatic cancer, cardiovascular disease
and type 2 diabetes. Even so, these risks appear to be much lower than those of smoking.
An expert panel estimated on the basis of the epidemiological literature that the overall risk
of tobacco-related mortality in LNSLT users was 10 % of the risk of cigarette smokers (Levy
et al., 2004). Epidemiological modelling of the aggregate health effects of quitting tobacco
and switching from smoking to LNSLT suggest there is little difference in years of healthy
life gained by those who quit tobacco and those who switch to LNSLT (Gartner et al.,
2007b) (see box on p. 262).
Effects of tobacco harm reduction on aggregate harm
Whether THR produces a net benefit or harm depends on: the relative harmfulness of the
THR product compared to regular cigarettes; how popular the THR product is among current
smokers, ex-smokers and never smokers; and its effect on rates of smoking cessation and
initiation. The risks of overall net harm are greatest for modified cigarettes and cigarette-like
devices, because these produce the least reduction in risk and could discourage cessation in
much the same way as ‘light’ cigarettes did.
Epidemiological modelling of the aggregate health effects of smoking and LNSLT use
suggests that relaxations of bans on LNSLT use would only produce net harm if these
products proved much more attractive to non-smokers than to smokers; led non-smokers to
start to smoke; and/or maintained cigarette use in smokers by dual use rather than complete
switching (Gartner et al., 2007a) (see box on p. 262). These putative effects of LNSLT have
not been observed in Sweden and there are good reasons for thinking that they are unlikely
to occur. As Kozlowski and colleagues (Kozlowski et al., 2001) have shown, PN would still
produce a net population health gain, even if we made: (1) the most pessimistic assumptions
about its residual health risks; and (2) we assumed that PN was used by the whole adult
population (Kozlowski et al., 2001). A similar argument can be made for LNSLT.
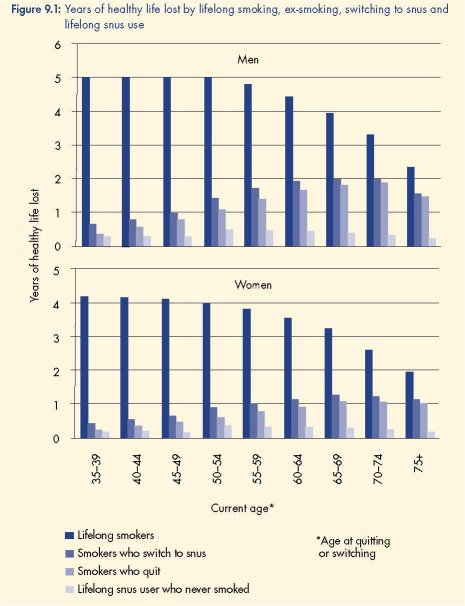
Epidemiological modelling of the aggregate health effects of lifelong smoking, ex-smoking,
switching to snus and lifelong snus use
Gartner et al (2007b) used multistate life tables and expert panel risk estimates to model the
years of healthy life lost (YHLL) due to lifelong smoking, quitting tobacco use, switching from
smoking to snus and lifelong snus use without smoking. The results showed that smokers who
switched to snus would achieve health gains nearly as good as quitting all tobacco use. Men
who switched from smoking to snus would lose 1.2–3.6 months of healthy life and women
1.2–4.8 months compared to smokers who quit tobacco altogether.
‘Gateway’ effects
There is no evidence that PN in its currently available forms encourages non-smokers to
take up smoking (Gerlach et al., 2008; Klesges et al., 2003). This situation could change if
PN was re-engineered to be more rapidly absorbed and produced higher blood nicotine,
and if it were allowed to be marketed as a recreational nicotine product, like smoked
tobacco. The current marketing of the e-cigarette in some countries may allow an
assessment of the risks of more liberal regulation of the nicotine market, although the
nicotine dose and delivery of currently marketed e-cigarettes may be too similar to existing
PN cessation aids for a full assessment. The cost of the e-cigarette may also preclude its
widescale uptake.
The Swedish experience
Snus is a traditional moist oral snuff used in Sweden. Snus use declined as cigarettes became
popular. However, a marketing campaign that started in the 1970s reinvigorated the snus
market and resulted in increased uptake among Swedish men, with as many Swedish men
now using snus as smoking cigarettes (Ramström, 2000). The Swedish experience has been
described as a natural experiment of tobacco harm reduction (Brandt, 2007; Henningfield
and Fagerström, 2001) as the shift from cigarette smoking to snus use has occurred without
the support of the Swedish health community.
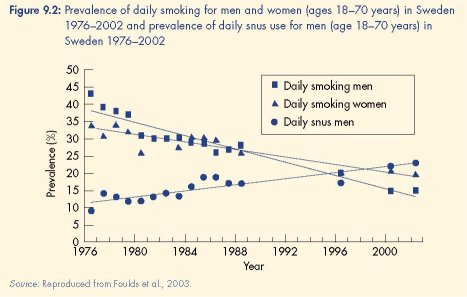
The increase in snus use was accompanied by a decline in cigarette smoking from 40 % in
1976 to 15 % in 2002 (see Figure 9.2). Contrary to the gateway hypothesis, there were no
increases in smoking among adolescent males, who were the heaviest users of snus. Instead,
snus use appears to deter smoking initiation in young men and promote smoking cessation in
older men (Foulds et al., 2003; Furberg et al., 2005; Ramström, 2000). Most critically, the
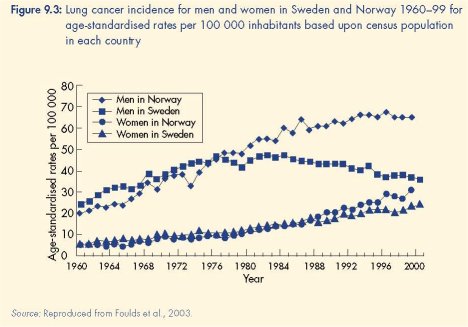
increase in snus use was accompanied by a decline in lung cancer mortality and the absence
of an increase in either cardiovascular mortality or head and neck cancers (Foulds et al.,
2003). The plausibility of a causal relationship between increased snus use and these good
health outcomes was strengthened by the absence of any similar changes in smoking
prevalence or lung cancer mortality in Swedish women, who did not adopt snus at the same
rate as men (Foulds et al., 2003).
Whether SLT serves as a gateway to smoking is a more contentious issue. The Swedish
experience with snus contradicts the pessimistic view about the population impact of THR
(Foulds et al., 2003) (see box on p. 263). The relationship between SLT use and smoking
has been more varied in American studies. In some studies the same pattern has been
reported as in Sweden (Ault et al., 2004; O’Connor et al., 2005). Other studies, however,
have reported an apparent ‘gateway’ effect with young SLT users ‘graduating’ to
smoking (Haddock et al., 2001). It is challenging to quantify how much smoking is
attributable to prior SLT use because it is difficult to determine whether smokers who used
SLT before cigarettes would have become smokers in the absence of SLT use. One
analysis suggests that when the demographic and social factors associated with smoking
initiation are taken into account, SLT does not appear to increase the uptake of smoking
(Timberlake et al., 2009). In the United States, public health authorities may have also
inadvertently encouraged SLT users to switch to cigarettes by claiming that the health
risks of SLT are the same as those of smoking (Kozlowski and Edwards, 2005; Kozlowski
and O’Connor, 2003; Waterbor et al., 2004).
‘Dual use’
The use of PN to relieve nicotine withdrawal during periods of temporary abstinence is an
approved use in some countries (for example, United Kingdom, Republic of Ireland, France,
Austria, Denmark, Norway, Portugal, Brazil, Venezuela, New Zealand and Canada), as is its
use to reduce smoking in preparation for quitting (ASH UK, 2008). Some studies have
reported that users of PN often use it for purposes other than cessation (Hammond et al.,
2008; Klesges et al., 2003). Such use does not appear to reduce quitting (Levy et al., 2007);
indeed, such use may increase cessation in smokers who were not initially interested in
quitting (Carpenter et al., 2004; Le Houezec and Sawe, 2003).
The tobacco industry has begun to market SLT for smokers to use when smoking is not
permitted (Gartner et al., 2007a). This pattern of use could perpetuate smoking by reducing
the incentive to quit provided by public smoking bans (Fichtenberg and Glantz, 2002).
Alternatively, such use of SLT could lead some smokers to switch fully to SLT or even to quit
tobacco use, as happens with PN. This pattern of short-term dual use as an intermediate step
to full switching or quitting appears more common in Sweden than long-term dual use of SLT
and cigarettes (Ramström and Foulds, 2006). It is a pattern that could be encouraged by a
combination of policies, such as educating smokers about health risks, imposing differential
tax rates on smoked tobacco and SLT products based on their relative harmfulness, and
regulating the availability and accessibility of these products to favour SLT.
Ethical issues
Do public health practitioners have the ethical right to prevent smokers from being informed
about THR products in order to reduce the possibility that THR may increase population
nicotine use? Those who argue that smokers should not be told how to reduce their risks
promote a paternalistic policy that sacrifices smokers’ interests to the greater public good.
Others argue that informing smokers about THR is an effective public health measure that
properly respects their autonomy (Kozlowski, 2003; Kozlowski and Edwards, 2005;
Waterbor et al., 2004).
Some opposition to THR reflects the belief that the goal of tobacco control policy should be the
elimination of all nicotine use (for example, Pierce, 2002). Some opponents also argue that THR
is morally wrong because it involves the long-term use of an addictive substance (Warner et al.,
1997). These views contrast with the consequentialist ethical views of proponents who argue
that the benefits of THR outweigh its harms (for example, Kozlowski, 2002).
The THR debate is complicated by the role of the tobacco industry whose interests conflict
with those of public health. THR is seen as benefiting the tobacco industry by condoning
continued tobacco use and thereby allowing the industry’s continued existence (Bullen et al.,
2006). Whilst the abolition of the tobacco industry would arguably be preferable, most THR
proponents see this as an unrealistic goal, at least in the short to medium term (Hall and
Gartner, 2009) and accept that enabling the tobacco industry to become part of the solution
could accelerate change in the nicotine market over time.
Options for promoting tobacco harm reduction
Regulating the harmfulness of tobacco products
Mandating standards for RIP of cigarettes is unlikely to cause harm and may reduce cigaretterelated
fires. It is much less certain whether mandated maximum levels of key toxins in cigarette
emissions will reduce aggregate harm because of the risk that any gains will be offset by
compensatory smoking, higher levels of other toxins, and/or the impression of a significant
reduction in harm. It will in any case take decades to assess. Mandated standards for toxins,
such as tobacco-specific nitrosamines, in SLT should be less problematic to implement because
the feasibility of this strategy has already been demonstrated (Österdahl et al., 2004; Stepanov
et al., 2006) and, on Swedish experience, it is likely to minimise oral cancer risk.
Information about THR products
Harm reduction could be promoted through advising smokers to use less harmful products,
such as LNSLT and PN. This could be done via product warning labels on cigarettes and less
harmful tobacco and nicotine products that indicate the relative harmfulness of each product.
This option is currently most relevant for non-EU countries and Sweden because of the sales
ban on most of these products in EU Member States. Information provided by governments
and health authorities could also clearly indicate the relative harms of each product, rather
than misleadingly suggesting that all tobacco products are equally hazardous (Kozlowski,
2003; Kozlowski and O’Connor, 2003; Waterbor et al., 2004).
Regulation and promotion of THR products
Smokers who fail to quit after obtaining cessation assistance could be encouraged to use PN
as a long-term alternative (Kozlowski, 2002; Kozlowski et al., 2003). This is one of the few
THR strategies supported by the majority of US tobacco control advocates (Warner and
Martin, 2003) and advocated by the Royal College of Physicians in the United Kingdom
(Royal College of Physicians, 2007) and experts in the EU (ASPECT Consortium, 2004). It
would probably have limited public health impact if it was aimed solely at high-risk smokers
who failed to quit, because only a minority of these smokers seek help to quit, and probably
few of whom find existing forms of PN attractive (Stratton et al., 2001; Warner et al., 1997).
In order to have a larger public health impact, THR requires as many smokers as possible to
switch to either PN or LNSLT. The Swedish experience suggests that LNSLT may be more likely
to achieve this goal than current forms of PN as more smokers in Sweden have switched to
LNSLT than PN (Foulds et al., 2003; Ramström, 2000). This could change if regulators allowed
more attractive forms of PN to be developed and marketed to smokers. In EU countries other
than Sweden, consideration could be given to relaxing the sales ban on non-smoked, nonchewed
oral tobacco products. More equal competition between cigarettes and less hazardous
nicotine delivery devices could be achieved by making it harder to introduce new cigarette-like
tobacco products and easier to introduce and promote the use of non-smoked THR products
and recreational PN products (Stratton et al., 2001; Warner et al., 1997). Thought should be
given to the regulation of products that fall between current PN products and cigarettes. The
e-cigarette could provide a test case for developing a more flexible regulatory structure that
works in favour of public health, by regulating nicotine-containing products according to
criteria that consider the relative harmfulness of each product.
A graduated policy sequence
We believe that exploring the use of LNSLT for THR is the most promising route facing
regulators at the moment. The development of faster-acting PN is likely to take some time and
e-cigarettes are probably too similar to PN products. The following steps could be used to
explore the public health potential of THR using LNSLT in those countries in which their
production and sale is prohibited, such as the EU, Australia and New Zealand
(Commonwealth of Australia, 1974; European Parliament and Council of the European Union,
2001; Parliament of New Zealand, 1990).
First, the utility of LNSLT for smoking cessation could be cautiously trialled among smokers who
had failed to quit with the use of PN and other smoking cessation medications by encouraging
them to switch to LNSLT rather than return to smoking. Evaluations of this approach would
provide information on how attractive these products may be to inveterate smokers.
Second, relaxation of PN product regulation could encourage the use of existing PN for longterm
substitution if smokers fail to stop, and enable the delivery of nicotine doses in ways
more like SLT, thereby encouraging smokers who failed to quit smoking to use these products
instead.
Third, if there was sufficient interest in switching to LNSLT among inveterate smokers,
permitting restricted sale of LNSLT products to these smokers (e.g. from specialist tobacconists)
could provide an alternative to continued smoking. Legislation could impose differential taxes
to reflect the relative harmfulness.
Fourth, the impacts of the sale of these products on: population smoking cessation rates; all
forms of tobacco use among youth; and tobacco industry marketing should be rigorously
evaluated.
Conclusions
Public smoking bans and mandatory reduced ignition propensity standards for cigarettes are
strategies that reduce tobacco-related harm to non-smokers and should be implemented as a
priority. The most promising strategy for reducing harm to tobacco smokers is to encourage
smokers who are unable or unwilling to quit to switch to pharmaceutical nicotine or low
nitrosamine smokeless tobacco products. There is good support for this policy from
epidemiological studies in Sweden. Modelling studies indicate that this would very
substantially reduce the risks of tobacco use. Nonetheless, this remains a controversial policy
because the view of some in the tobacco control community is that our policy goal should be
elimination of all nicotine use. A major barrier to its implementation is that many states in the
EU ban the sale of these products, and proposals to remove these bans have been opposed
because of concerns that THR may increase the uptake of tobacco smoking and the harm
that it causes.
References
Alpert, H. R., Carpenter, C., Connolly, G. N., Rees, V. and Wayne, G. F. (2005), ‘Fire safer’ cigarettes: the effect of
the New York State Cigarette Fire Safety Standard on ignition propensity, smoke toxicity, and the consumer market. A
preliminary report, Harvard School of Public Health, Boston. Available at http://www.firesafecigarettes.org/
assets/files/harvardstudy.pdf.
Arendt, P. (2008), ‘One month of ... electronic cigarettes’, Guardian, 8 December, Comment and features, p. 17.
Arnott, D. and Berteletti, F. (2008), ‘Europe: agreement on reducing cigarette fires’, Tobacco Control 17, pp. 4–5.
ASH UK (2008), Beyond Smoking Kills: protecting children, reducing inequalities, Action on Smoking and Health,
London. Available at www.ash.org.uk/beyondsmokingkills.
ASPECT Consortium (2004), Tobacco or health in the European Union, European Commission, Luxembourg.
Available at http://ec.europa.eu/health/ph_determinants/life_style/Tobacco/Documents/tobacco_fr_en.pdf.
Ault, R. W., Ekelund, R. B., Jackson, J. D. and Saba, R. P. (2004), ‘Smokeless tobacco, smoking cessation and
harm reduction: an economic analysis’, Applied Economics 36, pp. 17–29.
Benowitz, N. (2008), ‘Clinical pharmacology of nicotine: implications for understanding, preventing, and treating
tobacco addiction’, Clinical Pharmacology & Therapeutics 83, pp. 531–41.
Benowitz, N. L. (2000), ‘Nicotine toxicity’, in Ferrence, R., Slade, J., Room, R. and Pope, M. (eds), Nicotine and
public health, American Public Health Association, Washington, DC, pp. 65–76.
Berridge, V. (2007), Marketing health: smoking and the discourse of public health in Britain, 1945–2000, Oxford
University Press, Oxford.
Bertram, M. Y., Lim, S. S., Wallace, A. L. and Vos, T. (2007), ‘Costs and benefits of smoking cessation aids:
making a case for public reimbursement of nicotine replacement therapy in Australia’, Tobacco Control 16, pp.
255–60.
Brandt, A. (2007), The cigarette century: the rise, fall, and deadly persistence of the product that defined America,
Basic Books, New York.
Breland, A. B., Evans, S. E., Buchhalter, A. R. and Eissenberg, T. (2002), ‘Acute effects of AdvanceTM: a potential
reduced exposure product for smokers’, Tobacco Control 11, pp. 376–8.
Breland, A. B., Kleykamp, B. A. and Eissenberg, T. (2006), ‘Clinical laboratory evaluation of potential reduced
exposure products for smokers’, Nicotine & Tobacco Research 8, pp. 727–38.
Broadstock, M. (2007), Systematic review of the health effects of modified smokeless tobacco products, New Zealand
Health Technology Assessment Report 10/1, Department of Public Health and General Practice, Christchurch
School of Medicine and Health Sciences, Christchurch. Available at http://nzhta.chmeds.ac.nz/publications/
smokeless_tobacco.pdf.
Bullen, C., McRobbie, H., Thornley, S., Walker, N. and Whittaker, R. (2006), ‘Working with what we have before
getting into bed with the tobacco industry’, New Zealand Medical Journal 119, p. U2139.
Burns, D. M., Dybing, E., Gray, N., et al. (2008), ‘Mandated lowering of toxicants in cigarette smoke: a
description of the World Health Organization TobReg proposal’, Tobacco Control 17, pp. 132–41.
Carpenter, M. J., Hughes, J. R., Solomon, L. J. and Callas, P. W. (2004), ‘Both smoking reduction with nicotine
replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit’,
Journal of Consulting and Clinical Psychology 72, pp. 371–81.
Chapman, S. and Balmain, A. (2004), Reduced-ignition propensity cigarettes: a review of policy relevant information
prepared for the Commonwealth Department of Health and Ageing, Commonwealth of Australia, Canberra.
Available at http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pubhlth-publicat-documentsmoking_
rip.htm.
Chapman, S. and Freeman, B. (2008), ‘Markers of the denormalisation of smoking and the tobacco industry’,
Tobacco Control 17, pp. 25–31.
Commission of the European Communities (2008), Commission Decision of 25 March 2008 on the fire safety
requirements to be met by European standards for cigarettes pursuant to Directive 2001/95/EC of the European
Parliament and of the Council, Official Journal of the European Union, Office for Official Publications of the
European Communities, Luxembourg. Available at http://eur-lex.europa.eu/.
Commonwealth of Australia (1974), Trade Practices Act No. 51 of 1974, Attorney General’s Department. Available
at http://www.comlaw.gov.au/.
Danish Medicines Agency (2009), Classification of electronic cigarettes, March 9 2009 (medicinal products),
Lægemiddelstyrelsen, Copenhagen. Available at http://www.dkma.dk/1024/visUKLSArtikel.
asp?artikelID=14819.
European Commission Health and Consumer Protection Directorate-General (2008), Orientation note: electronic
cigarettes and the EC legislation, European Commission, Brussels. Available at http://ec.europa.eu/health/ph_
determinants/life_style/Tobacco/Documents/orientation_0508_en.pdf.
European Court of Justice (2004), ‘The Court declares the prohibition on tobacco products for oral use to be
valid’, press release No. 99/04, Judgments of the Court of Justice in Cases C-210/03 and C-434/02, European
Court of Justice, Luxembourg. Available at http://curia.europa.eu/.
European Parliament and Council of the European Union (2001), Directive 2001/37/EC of the European Parliament
and of the Council of 5 June 2001 on the approximation of the laws, regulations and administrative provisions of the
Member States concerning the manufacture, presentation and sale of tobacco products, Official Journal of the
European Union, Luxembourg. Available at http://eur-lex.europa.eu/.
Fagerström, K. O. and Jiménez-Ruiz, C. A. (2008), ‘Pharmacological treatments for tobacco dependence’,
European Respiratory Review 17, pp. 192–8.
Fagerström, K. O., Hughes, J. R., Rasmussen, T. and Callas, P. W. (2000), ‘Randomised trial investigating effect
of a novel nicotine delivery device (Eclipse) and a nicotine oral inhaler on smoking behaviour, nicotine and
carbon monoxide exposure, and motivation to quit’, Tobacco Control 9, pp. 327–33.
Fichtenberg, C. M. and Glantz, S. A. (2002), ‘Effect of smoke-free workplaces on smoking behaviour: systematic
review’, British Medical Journal 325, pp. 188–95.
Foulds, J., Ramström, L., Burke, M. and Fagerström, K. (2003), ‘Effect of smokeless tobacco (snus) on smoking and
public health in Sweden’, Tobacco Control 12, pp. 349–59.
Furberg, H., Bulik, C. M., Lerman, C., et al. (2005), ‘Is Swedish snus associated with smoking initiation or
smoking cessation?’ Tobacco Control 14, pp. 422–4.
Gartner, C. E., Hall, W. D., Chapman, S. and Freeman, B. (2007a), ‘Should the health community promote
smokeless tobacco (snus) as a harm reduction measure?’, PLoS Medicine 4, pp. 1703–04.
Gartner, C. E., Hall, W. D., Vos, T., et al. (2007b), ‘Assessment of Swedish snus for tobacco harm reduction: an
epidemiological modelling study’, Lancet 369, pp. 2010–14.
Gartner, C., Barendregt, J. and Hall, W. (2009), ‘Predicting the future prevalence of cigarette smoking in
Australia: how low can we go and by when?’ Tobacco Control 18, pp. 183–9.
Gerlach, K. K., Rohay, J. M., Gitchell, J. G. and Shiffman, S. (2008), ‘Use of nicotine replacement therapy among
never smokers in the 1999–2006 National Health and Nutrition Examination Surveys’, Drug and Alcohol
Dependence 98, pp. 154–8.
Glantz, S. A., Slade, J., Bero, L. A., Hanauer, P. and Barnes, D. E. (eds) (1996), The cigarette papers, University of
California Press, Berkeley.
Gray, N. (2004), ‘The ethics of policies for the prevention of tobacco disease’, Acta Oncologica 43, pp. 8–10.
Gunja, M., Wayne, G. F., Landman, A., Connelly, G. and McGuire, A. (2002), ‘The case for fire safe cigarettes
made through industry documents’, Tobacco Control 11, pp. 346–53.
Haddock, C. K., Vander Weg, M., DeBon, M., et al. (2001), ‘Evidence that smokeless tobacco use is a gateway
for smoking initiation in young adult males’, Preventive Medicine 32, pp. 262–7.
Hajek, P., McRobbie, H. and Gillison, F. (2007), ‘Dependence potential of nicotine replacement treatments:
effects of product type, patient characteristics, and cost to user’, Preventive Medicine 44, pp. 230–4.
Hall, W. D. and Gartner, C. E. (2009), ‘Supping with the devil? The role of law in promoting tobacco harm
reduction using low nitrosamine smokeless tobacco products’, Public Health 123, pp. 287–91.
Hammond, D., Reid, J. L., Driezen, P., et al. (2008), ‘Smokers’ use of nicotine replacement therapy for reasons
other than stopping smoking: findings from the ITC Four Country Survey’, Addiction 103, pp. 1696–703.
Hatsukami, D. K., Lemmonds, C., Zhang, Y., et al. (2004), ‘Evaluation of carcinogen exposure in people who
used “reduced exposure” tobacco products’, Journal of the National Cancer Institute 96, pp. 844–52.
Hatsukami, D. K., Ebbert, J. O., Feuer, R. M., Stepanov, I. and Hecht, S. S. (2007), ‘Changing smokeless tobacco
products: new tobacco delivery systems’, American Journal of Preventive Medicine 33, pp. S368–S78.
Henningfield, J. E. and Fagerström, K. O. (2001), ‘Swedish match company, Swedish snus and public health: a
harm reduction experiment in progress?’, Tobacco Control 10, pp. 253–7.
Hoffmann, D., Djordjevic, M. V., Fan, J., et al. (1995), ‘Five leading U.S. commercial brands of moist snuff in
1994: assessment of carcinogenic N-nitrosamines’, Journal of the National Cancer Institute 87, pp. 1862–9.
Hopkins, D. P., Briss, P. A., Ricard, C. J., et al. (2001), ‘Reviews of evidence regarding interventions to reduce
tobacco use and exposure to environmental tobacco smoke’, American Journal of Preventive Medicine 20, pp.
16–66.
Joossens, L. and Raw, M. (2007), Progress in tobacco control in 30 European countries, 2005 to 2007, Swiss
Cancer League, Berne. Available at http://www.ensp.org/files/30_european_countries_text_final.pdf.
Kemm, J. (2003), ‘A model to predict the results of changes in smoking behaviour on smoking prevalence’,
Journal of Public Health Medicine 25, pp. 318–24.
King, B., Borland, R. and Fowles, J. (2007), ‘Mainstream smoke emissions of Australian and Canadian cigarettes’,
Nicotine & Tobacco Research 9, pp. 835–44.
Klesges, L. M., Johnson, K. C., Somes, G., Zbikowski, S. and Robinson, L. (2003), ‘Use of nicotine replacement
therapy in adolescent smokers and nonsmokers’, Archives of Pediatrics and Adolescent Medicine 157, pp. 517–22.
Kozlowski, L. T. (2002), ‘Harm reduction, public health, and human rights: smokers have a right to be informed of
significant harm reduction options’, Nicotine & Tobacco Research 4, pp. S55–S60.
Kozlowski, L. T. (2003), ‘First, tell the truth: a dialogue on human rights, deception, and the use of smokeless
tobacco as a substitute for cigarettes’, Tobacco Control 12, pp. 34–6.
Kozlowski, L. T. and Edwards, B. Q. (2005), ‘“Not safe” is not enough: smokers have a right to know more than
there is no safe tobacco product’, Tobacco Control 14, pp. II3–II7.
Kozlowski, L. T. and O’Connor, R. J. (2003), ‘Apply federal research rules on deception to misleading health
information: an example on smokeless tobacco and cigarettes’, Public Health Reports 118, pp. 187–92.
Kozlowski, L. T., Strasser, A. A., Giovino, G. A., Erickson, P. A. and Terza, J. V. (2001), ‘Applying the risk/use
equilibrium: use medicinal nicotine now for harm reduction’, Tobacco Control 10, pp. 201–03.
Kozlowski, L. T., O’Connor, R. J. and Quinio Edwards, B. (2003), ‘Some practical points on harm reduction: what
to tell your lawmaker and what to tell your brother about Swedish snus’, Tobacco Control 12, pp. 372–3.
Laugesen, M. (2008), Second safety report on the Ruyan® e-cigarette, Health New Zealand Ltd, Christchurch.
Available at http://www.healthnz.co.nz/2ndSafetyReport_9Apr08.pdf.
Laugesen, M., Thornley, S., McRobbie, H. and Bullen, C. (2008), How safe is an e-cigarette? The results of
independent chemical and microbiological analysis (poster), SRNT 14th Annual Meeting, Society for Research on
Nicotine and Tobacco, Portland, Oregon.
Le Houezec, J. and Sawe, U. (2003), ‘Smoking reduction and temporary abstinence: new approaches for
smoking cessation’, Journal des Maladies Vasculaires 28, pp. 293–300.
Levy, D. T., Mumford, E. A., Cummings, K. M., et al. (2004), ‘The relative risks of a low-nitrosamine smokeless
tobacco product compared with smoking cigarettes: estimates of a panel of experts’, Cancer Epidemiology,
Biomarkers and Prevention 13, pp. 2035–42.
Levy, D. E., Thorndike, A. N., Biener, L. and Rigotti, N. A. (2007), ‘Use of nicotine replacement therapy to reduce
or delay smoking but not to quit: prevalence and association with subsequent cessation efforts’, Tobacco Control
16, pp. 384–9.
Medioni, J., Berlin, I. and Mallet, A. (2005), ‘Increased risk of relapse after stopping nicotine replacement
therapies: a mathematical modelling approach’, Addiction 100,
pp. 247–54.
Mendez, D., Warner, K. E. and Courant, P. N. (1998), ‘Has smoking cessation ceased? Expected trends in the
prevalence of smoking in the United States’, American Journal of Epidemiology 148, pp. 249–58.
Murray, R. P., Bailey, W. C., Daniels, K., et al. (1996), ‘Safety of nicotine polacrilex gum used by 3,094
participants in the Lung Health Study. Lung Health Study Research Group’, Chest 109, pp. 438–45.
National Drugs and Poisons Scheduling Committee (2009), Standard for the uniform scheduling of drugs and
poisons (SUSDP) No. 23, Department of Health and Ageing, Commonwealth of Australia, Canberra. Available at
http://www.comlaw.gov.au/.
New Zealand Public Health Directorate (2006), ‘Classification of medicines notice, schedule 3, pharmacy-only
medicines: nicotine’, New Zealand Gazette, NZ Department of Internal Affairs, Wellington, p. 188.
Nides, M. (2008), ‘Update on pharmacologic options for smoking cessation treatment’, American Journal of
Medicine 121, pp. S20–S31.
O’Connor, R. J., Kozlowski, L. T., Flaherty, B. P. and Edwards, B. Q. (2005), ‘Most smokeless tobacco use does
not cause cigarette smoking: results from the 2000 National Household Survey on Drug Abuse’, Addictive
Behaviors 30, pp. 325–36.
Österdahl, B. G., Jansson, C. and Paccou, A. (2004), ‘Decreased levels of tobacco-specific N-nitrosamines in
moist snuff on the Swedish market’, Journal of Agricultural and Food Chemistry 52, pp. 5085–8.
Pankow, J. F., Watanabe, K. H., Toccalino PL, Luo, W. and Austin, D. F. (2007), ‘Calcuated cancer risk for
conventional and “potentially reduced exposure product” cigarettes’, Cancer Epidemiology, Biomarkers and
Prevention 16, pp. 584–92.
Parliament of New Zealand (1990), Smoke-free environments act no. 108. Available at http://www.legislation.
govt.nz/act/public/1990/0108/latest/DLM223191.html?search=ts_act_smoke-free+environments&;sr=1.
Pell, J. P., Haw, S., Cobbe, S., et al. (2008), ‘Smoke-free legislation and hospitalizations for acute coronary
syndrome’, New England Journal of Medicine 359, pp. 482–91.
Pierce, J. P. (2002), ‘Harm reduction or harm maintenance?’, Nicotine and Tobacco Research 4, pp. S53–S4.
Ramström, L. (2003), ‘Snus: part of the problem or part of the solution?’, Addiction 98, pp. 1198–9.
Ramström, L. M. (2000), ‘Snuff: an alternative nicotine delivery system’, in Ferrence, R., Slade, J., Room, R. and
Pope, M. (eds), Nicotine and public health, American Public Health Association, Washington, DC.
Ramström, L. M. and Foulds, J. (2006), ‘Role of snus in initiation and cessation of tobacco smoking in Sweden’,
Tobacco Control 15, pp. 210–4.
Rees, V. W., Wayne, G. F. and Connolly, G. N. (2008), ‘Puffing style and human exposure minimally altered by
switching to a carbon-filtered cigarette’, Cancer Epidemiology Biomarkers & Prevention 17, p. 2995.
Royal College of Physicians (2007), Harm reduction in nicotine addiction: helping people who can’t quit. A report by
the Tobacco Advisory Group of the Royal College of Physicians, RCP, London. Available at http://www.rcplondon.
ac.uk/.
SCENIHR (2008), Health effects of smokeless tobacco products, Scientific Committee on Emerging and Newly
Identified Health Risks, European Commission, Brussels. Available at http://ec.europa.eu/health/ph_risk/
committees/04_scenihr/docs/scenihr_o_013.pdf.
Shiffman, S., Gitchell, J. G., Warner, K. E., et al. (2002a), ‘Tobacco harm reduction: conceptual structure and
nomenclature for analysis and research’, Nicotine & Tobacco Research 4, pp. S113–S129.
Shiffman, S., Rolf, C. N., Hellebusch, S. J., et al. (2002b), ‘Real world efficacy of prescription and over-thecounter
nicotine replacement therapy’, Addiction 97, pp. 505–16.
Sikora, K. (2007), ‘Electric cigarette beats pub smoking ban’, Daily Telegraph 15 November. Available at http://
www.news.com.au/story/0,23599,22762416-13762,00.html.
Stead, L. F., Perera, R., Bullen, C., Mant, D. and Lancaster, T. (2008), ‘Nicotine replacement therapy for smoking
cessation’, Cochrane Database of Systematic Reviews, CD000146.
Stepanov, I., Jensen, J., Hatsukami, D. and Hecht, S. S. (2006), ‘Tobacco-specific nitrosamines in new tobacco
products’, Nicotine & Tobacco Research 8, pp. 309–13.
Stratton, K., Shetty, P., Wallace, R. and Bondurant, S. (eds) (2001), Clearing the smoke: assessing the science base
for tobacco harm reduction, National Academy Press, Washington, DC.
Timberlake, D. S., Huh, J. and Lakon, C. M. (2009), ‘Use of propensity score matching in evaluating smokeless
tobacco as a gateway to smoking’, Nicotine and Tobacco Research 11, pp. 455–62.
Toumbourou, J. W., Stockwell, T., Neighbors, C., et al. (2007), ‘Adolescent health 4: interventions to reduce harm
associated with adolescent substance use’, Lancet 369, pp. 1391–401.
US Department of Health and Human Services (2006), The health consequences of involuntary exposure to tobacco
smoke: a report of the Surgeon General, U.S. Department of Health and Human Services, Centers for Disease
Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on
Smoking and Health, Atlanta, GA. Available at http://www.surgeongeneral.gov/library/secondhandsmoke/.
Warner, K. E. (2001), ‘Reducing harm to smokers: methods, their effectiveness and the role of policy’, in Rabin, R.
L. and Sugarman, S. D. (eds), Regulating tobacco, Oxford University Press, Oxford, pp. 111–42.
Warner, K. E. and Martin, E. G. (2003), ‘The US tobacco control community’s view of the future of tobacco harm
reduction’, Tobacco Control 12, pp. 383–90.
Warner, K. E., Slade, J. and Sweanor, D. T. (1997), ‘The emerging market for long-term nicotine maintenance’,
JAMA 278, pp. 1087–92.
Waterbor, J. W., Adams, R. M., Robinson, J. M., Crabtree, F. G., Accortt, N. A. and Gilliland, M. J. (2004),
‘Disparities between public health educational materials and the scientific evidence that smokeless tobacco use
causes cancer’, Journal of Cancer Education 19, pp. 17–28.
WHO Regional Office for Europe (2003), WHO European country profiles on tobacco control, World Health
Organization, Copenhagen. Available at http://www.euro.who.int/tobaccofree/publications/publications.
WHO Regional Office for Europe (2007), The European tobacco control report 2007, World Health Organization,
Copenhagen. Available at http://www.euro.who.int/tobaccofree/publications/publications.
World Bank (2003), Tobacco control at a glance, World Bank Group, Washington, DC. Available at http://go.
worldbank.org/3HHPVQI020.
| < Prev | Next > |
|---|












