Chapter 6 The effect of epidemiological setting on the impact of harm reduction targeting injecting drug users
| Reports - EMCDDA Harm Reduction |
Drug Abuse
Chapter 6 The effect of epidemiological setting on the impact of harm reduction targeting injecting drug users
Peter Vickerman and Matthew Hickman
Abstract
Hepatitis C (HCV) and HIV cause substantial morbidity and mortality, and are both easily transmitted through contaminated syringes. Although reducing the transmission of HCV and HIV through injecting drug use is critical to preventing both infections, there is little evidence that interventions targeting injecting drug users (IDUs) reduce the transmission of either HIV or HCV.
A recent systematic review suggested a strong positive relationship between the prevalence of HIV and HCV in different IDU populations, but with considerable variability in different settings. This analysis uses a dynamic HIV and HCV transmission model to investigate the possible reasons for these observed trends, and to explore whether HIV and/or HCV prevalence could be used as proxy markers for the relative impact of an IDU intervention in different settings. By varying the HIV and HCV transmission probabilities and other nonsetting specific HIV/HCV natural history parameters, a dynamic compartmental model of HIV and HCV transmission was fitted, to observe trends in HIV and HCV prevalence from different settings. Using multivariate linear regression, the output from the ‘best-fitting’ simulated epidemics was used to identify factors that determine the type of HIV and HCV epidemic that occurs. These simulated epidemics were then used to project the impact of a generic intervention that reduced syringe sharing amongst all IDUs or just low- or high-risk IDUs, and to explore whether the impact projections correlated with HIV and/or HCV prevalence.
Results showed that the relative HCV to HIV transmission probability was the main factor determining how well the model agreed with the observed HIV and HCV prevalence trends. The ‘best-fitting’ model projections suggest that the relative HIV to HCV prevalence in different epidemic scenarios is positively correlated to the relative proportion of and level of risk behaviour among the high-risk IDUs. Indeed, the projected impact of the generic intervention was also strongly correlated with the baseline HIV and HCV prevalence of the simulated epidemic, with more impact (HIV/HCV infections averted and relative decrease in HIV/HCV incidence) occurring in higher HCV prevalence settings but less impact occurring, except for HIV infections averted, in higher baseline HIV prevalence settings. Lastly, a generic intervention among IDUs had less impact on the HCV epidemic compared to the HIV epidemic in most scenarios. However, when the intervention reached only lower-risk IDUs, it had only little impact on the HIV epidemic and greater impact on the HCV epidemic.
We conclude that the trends and variability in HIV and HCV prevalence observed in different epidemiological settings may be mainly due to differences in the heterogeneity of IDU risk behaviour. The HIV and HCV prevalence in a setting could be proxy markers for the relative impact of an intervention.
Keywords: injecting drug use, HIV, hepatitis C, mathematical modelling.
Introduction
Hepatitis C (HCV) and HIV cause substantial morbidity and mortality. Worldwide, 40 million people are estimated to be infected with HIV (UNAIDS, 2004a) and 170 million with HCV (WHO, 2000a). HCV and HIV can easily be transmitted through contaminated syringes (Baggaley et al., 2006; De Carli et al., 2003), and, while HIV infection rates vary widely (UNAIDS, 2004b), HCV infection rates are often very high amongst injecting drug users (IDUs) (Health Protection Agency, 2004; Wiessing and Nardone, 2006). Similarly to HIV, HCV infection is an important public health concern in Europe and elsewhere, because the majority of HCV infections do not resolve (Micallef et al., 2006) but become chronic and over time lead to cirrhosis of the liver, and possibly liver cancer and death (Limburg, 2004).
A worldwide systematic ecological analysis by the authors has shown that there is a strong positive relationship between the prevalence of HIV and that of HCV in different IDU populations, with the mean HIV prevalence generally being negligible if HCV prevalence is less than 30 % and thereafter increasing linearly with HCV prevalence (Vickerman et al., 2009a). Although this suggests that HCV prevalence could be used as a proxy indicator for injection-related HIV risk, there was substantial variability around the relationship when HCV prevalence is greater than 30 %, suggesting that other factors, such as the stage of the HIV epidemic and heterogeneity in IDU risk behaviour, may also play a role. This variability in HIV and HCV prevalence exists in all world regions, with the HIV prevalence in different European settings varying between 0 % and 70 % (Muga et al., 2007) and the HCV prevalence varying between 2.8 % (Danis et al., 2007) and 98 % (Westh et al., 1993). Overall, HIV prevalence in Europe is low; with high rates of prevalence being found in local studies. Nonetheless, despite this variability, the systematic review suggests that HCV prevalence could be used as a proxy indicator for injection-related HIV risk — and as a target or threshold level to minimise the possibility of an HIV outbreak.
Modelling health harm and its reduction
Reducing the transmission of HCV and HIV through injecting drug use is critical to the overall prevention of these infections in most countries. Epidemiological studies have shown that needle exchange and opioid substitution therapy (OST) interventions can reduce HIV transmission (Gibson et al., 1999; Gibson et al., 2001). However, the evidence for interventions impacting on HCV transmission is modest (Des Jarlais et al., 2005; Goldberg et al., 2001; van den Berg et al., 2007), with only one European study showing that full harm reduction (syringe distribution and OST) can reduce not only HIV incidence by 57 %, but also HCV incidence by 64 % (van den Berg et al., 2007). This lack of evidence is partly due to a relative dearth of epidemiological studies that estimate the impact of IDU interventions on HCV incidence, probably due to their high cost and difficulties in following up ‘chaotic’ IDUs (Craine et al., 2009). Although it is not the gold standard for evaluating interventions, modelling can play an important role in these difficult situations by translating intermediate intervention outcomes (HIV/HCV prevalence trends and/or even decreases in IDU risk behaviour) into projected decreases in HIV/HCV incidence or number of infections averted. Indeed, modelling can also answer ‘what if’ questions that would otherwise be very difficult to answer with epidemiological studies.
Numerous model analyses have provided important insights into the potential impact and costeffectiveness of different intervention strategies for IDUs in Europe, the United States, Eastern Europe, Asia and Australia (Blower et al., 1991; Kaplan and Heimer, 1994; Kretzschmar and Wiessing, 1998; Kretzschmar and Wiessing, 2008; Murray et al., 2003; Vickerman et al., 2006b; Vickerman et al., 2007). However, most studies have focused on HIV, with the small number of HCV modelling analyses being hampered by simplified epidemiology of HCV or IDU risk behaviour, or uncertainty surrounding key behavioural or biological parameters (Kretzschmar and Wiessing, 2004; Murray et al., 2003; Pollack, 2001; Vickerman et al., 2007).
Despite this, a number of analyses have fit HCV models to epidemiological data from one setting (Hutchinson et al., 2006; Murray et al., 2003; Vickerman et al., 2007; Vickerman et al., 2009b), mainly to estimate the impact of changes in syringe distribution/sharing. However, this model fitting strategy may not adequately calibrate an HCV transmission model, because one epidemic profile will contain insufficient information to fully understand the nature of HCV epidemics in other settings. This was emphasised in two studies by the first author of this chapter, which found that very different model parameterisations could accurately fit the HCV prevalence data from London, United Kingdom (Vickerman et al., 2007) or Rawalpindi, Pakistan (Vickerman et al., 2009b).
Few epidemiological or modelling analyses have considered the impact on the transmission of both HIV and HCV of an intervention targeting IDUs (Kwon et al., 2009; Murray et al., 2003; Vickerman et al., 2009b), and none have explored how impact could vary by the extent of baseline HIV and HCV epidemic occurring in a setting. In an attempt to fill this knowledge gap and to reduce the parametric uncertainty around modelling HCV, this analysis uses a joint HIV and HCV transmission mathematical model, fitted to the observed trends between HIV and HCV prevalence, to explore how the impact of a generic intervention (any one that reduces the extent of syringe sharing among IDUs) varies across different epidemiological scenarios. The model’s biological parameters are calibrated by determining which set of ‘biological parameters’ (HIV and HCV transmission and natural history parameters) most accurately produces the observed relationship between HIV and HCV prevalence when IDU behavioural parameters are widely varied to produce different epidemics. The simulated epidemics for this ‘biological’ parameter set are then used to explore how the impact of a generic intervention varies by HIV and HCV prevalence. More complex ‘realistic’ interventions (i.e., combinations of interventions, including treatment) were not modelled because it was not the main focus of the study, but impact estimates were made amongst high- and low-frequency syringe sharers in order to understand how targeted IDU interventions may differentially impact on HIV and HCV.
Methods
There were a number of stages to this analysis, many of which built on the work of previous analyses. First, the HIV and HCV transmission mathematical model and different ‘biological’ parameter sets (includes HIV and HCV transmission probabilities and non-setting specific natural history parameters — see Table 6.1 for all ‘biological’ parameters) that produced model fits in a recent modelling analysis from a specific IDU population (Vickerman et al., 2009b) were used as the basis for this analysis. For each of the biological parameter sets, an extensive uncertainty analysis was undertaken by randomly sampling specific IDU risk behaviour parameters (see parameters in Table 6.1) to produce 100 different behavioural parameter sets and so 100 different simulated HCV and HIV epidemics. These 100 behavioural parameter sets were chosen so that a wide variety of simulated HIV and HCV epidemics would be produced, and the same behavioural parameter sets were used for each biological parameter set. For each parameter set, the simulated epidemic was run until the overall HCV prevalence among IDUs was stable and the HIV epidemic had run for 30 years. The projected HIV/HCV prevalences were then compared to HIV and HCV prevalence trends for different IDU populations from a systematic review to see which biological parameter set produced the highest proportion of HIV and HCV epidemic projections lying within a defined area containing the vast majority of the paired HIV and HCV prevalence estimates from the systematic review. The 100 runs for this ‘best fit’ biological parameter set were used to explore how the impact of reducing the frequency of syringe sharing by 50 %, either amongst all IDUs or just among low-frequency (sharing syringes less often than once a week) or high-frequency (sharing syringes at least once a week) syringe sharers, varies for epidemics with different endemic HIV and HCV prevalence. Intervention impact was estimated over three years in terms of HIV and HCV infections averted (per 1 000 IDUs) and the relative decrease in HIV and HCV incidence (defined as the decrease of HIV and, respectively, HCV incidence compared to the baseline incidence).
Model derivation
A deterministic compartmental model of HCV and HIV transmission developed for a
previously published analysis was used (Vickerman et al., 2009b). In brief, the model
simulates the transmission of both HCV and HIV amongst IDUs with different levels of needle/
syringe sharing. The model includes three behavioural subgroups of IDUs depending on
whether they do not share needles/syringes (do not inject with a previously used needle/
syringe), or share with a low or high frequency. The model simulates the transmission of
HCV/HIV over time and includes two sub-groups for those that are new injectors and those
that have been injecting for longer. IDUs leave the population if they cease injecting, die or
experience severe HIV-related morbidity.
The HCV transmission model assumes that IDUs enter an acute phase of infection once
infected, and either resolve their infection after a number of months or progress to lifelong
chronic infection. A proportion of those that resolve HCV are assumed to become immune,
and the remainder become susceptible again (Aitken et al., 2008; Currie et al., 2008;
Dalgard, 2005; Grebely et al., 2006; Micallef et al., 2007). All infecteds develop an
antibody response during their acute phase.
The HIV transmission model assumes that once susceptible individuals are infected they
progress to a high viraemia phase of infection, following which they progress to a longer
stage of low viraemia, a short period of high viraemia pre-AIDS, and then AIDS. Because the
focus of the study was to look at the impact of interventions aiming to reduce injecting risks,
the model did not simulate the sexual transmission of HIV.
The HCV and HIV models are run in parallel once the HCV transmission model has
reached a stable state without HIV. From that point, the model follows the HIV/HCV coinfection
state of each IDU and assumes that being HIV infected exacerbates the effects of
being HCV infected, both in terms of the secondary transmission and the natural history of
HCV. Because of evidence that HIV infection increases both the HCV viral load in coinfected
IDUs (Bonacini et al., 2001; Daar et al., 2001; Fishbein et al., 2006; Thomas et al.,
2000b; Thomas et al., 2001) and the probability of mother-to-child HCV transmission
(Pappalardo, 2003), the HCV secondary transmission probability was assumed to be
heightened in HIV/HCV co-infected IDUs. In addition, it was assumed that the probability
that an HCV infection resolves was reduced in HIV/HCV co-infected individuals (Bonacini
et al., 2001; Daar et al., 2001; Grebely et al., 2007; Mehta et al., 2002; Thomas et al.,
2000a), and so was the probability that they develop immunity against HCV (Grebely et
al., 2006; Mehta et al., 2002).
This HIV/HCV prevalence model also has an additional component that incorporates the
possible effect of a generic intervention. After a certain time the intervention reduces the
frequency of syringe sharing amongst IDUs, regardless of whether they share syringes with a
low or high frequency, and affects the epidemiology of HIV and HCV in the modelled
population.
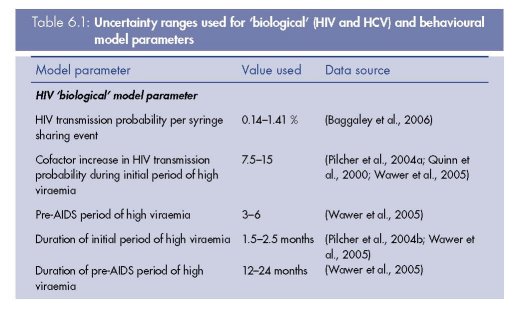
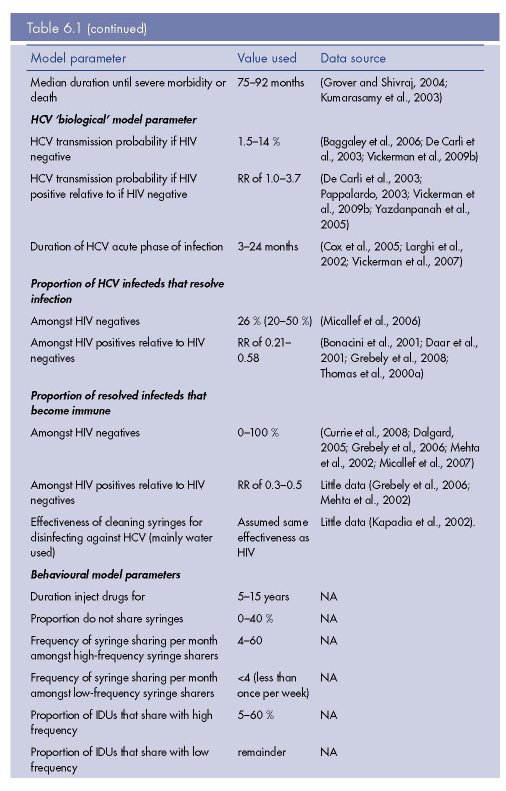
Model parameter values used for model fitting
The model was parameterised using different biological parameter sets that produced
model fits (n=42) to HIV and HCV prevalence data from a specific IDU population
(Vickerman et al., 2009b). These parameter sets were obtained during a rigorous fitting
process that also sampled across the uncertainty ranges for the behavioural parameters
from that setting. The behavioural parameters were mainly obtained from an in-depth
survey undertaken in the setting. The 42 biological model parameter sets were from a
total of 400 non-setting specific parameter sets that were randomly sampled from
parameter uncertainty ranges during the model fitting process. The parameter uncertainty
ranges for the biological parameters were obtained from the literature, and included such
aspects as the HIV and HCV transmission probabilities, the duration of the HCV acute
phase, and proportion of HCV infecteds that resolve infection. See Table 6.1 for the
biological parameters and their uncertainty ranges. All parameters had similar ranges
amongst the model fits from the previous analysis, except for the HIV and HCV
transmission probabilities, which had the ranges of 0.34–1.4 % and 1.5–5.0 %,
respectively.
Figure 6.1: Weighted HIV and HCV prevalence data from 310 different IDU populations including
the defined region for determining whether a model simulation is a model fit or not
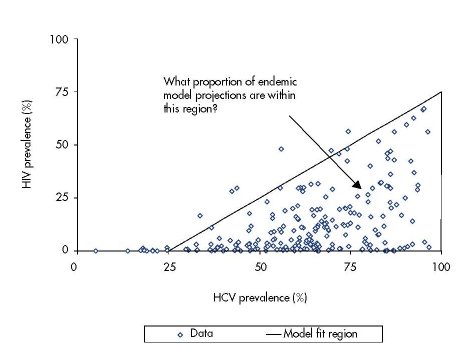
One hundred behavioural parameter sets were randomly sampled from the behavioural
parameter uncertainty ranges in Table 6.1, and these parameter sets were used to
simulate 100 different HIV and HCV epidemics for each biological parameter set. Each
epidemic was run until the HCV prevalence changed by <0.05 % in one year and then
the HIV epidemic was run for 30 years. The ranges for each behavioural parameter
were set to allow the model to produce a wide range of different HIV and HCV
epidemics.
For each biological parameter set, the 100 model simulations were compared against
collated HIV and HCV prevalence data from different IDU cross-sectional surveys found
by our systematic review (Vickerman et. al, 2009a) (see Figure 6.1). This systematic review
obtained weighted HIV and HCV prevalence estimates for 310 different IDU populations
from peer reviewed journal articles, international AIDS conference abstracts between
2000–08, the European IDU HIV/HCV database (managed by the European Monitoring
Centre for Drugs and Drug Addiction) (EMCDDA, 2008), United Kingdom unlinked
prevalence monitoring programme (managed by the Health Protection Agency) (Health
Protection Agency, 2008), and WHO Multi-City Drug Injection Study Phase II (WHO,
2000b). A model simulation was defined as a fit to the collated HIV/HCV prevalence data
if the last time point of the model simulation projected an HIV and HCV prevalence within
the region surrounded by a bolded triangle shown in Figure 6.1. The percentage of model
runs that were model fits for a particular biological parameter set was used to evaluate its
goodness of fit. The model projections for the best-fitting biological parameter set were
also validated against available HIV and HCV incidence data from different IDU
populations (obtained through a non-systematic literature review) to confirm that the
model produced a similar relationship between HIV/HCV incidence and HIV/HCV
prevalence.
Intervention impact projections
The biological parameter set with the best goodness of fit was used to explore how the
impact of a 50 % reduction in the frequency of syringe sharing will vary by endemic HIV and
HCV prevalence in different epidemic settings. A 50 % reduction in syringe sharing was
chosen for illustrative purposes, but this figure also reflects an upper bound estimate for what
can be achieved with intensive needle and syringe distribution (Foss et al., 2007; Hutchinson
et al., 2006). The impact of the intervention was estimated in terms of HIV/HCV infections
averted (per 1 000 IDUs) and the relative decrease in HIV/HCV incidence over three years.
Scatter plots, partial correlation coefficients and linear regression models (with ‘impact’ as
the independent variable and HIV and HCV prevalence as the dependent variables) were
used to assess the relationship between the variables, and the R-squared statistic was used to
determine the strength of the association.
Insights from model fitting process
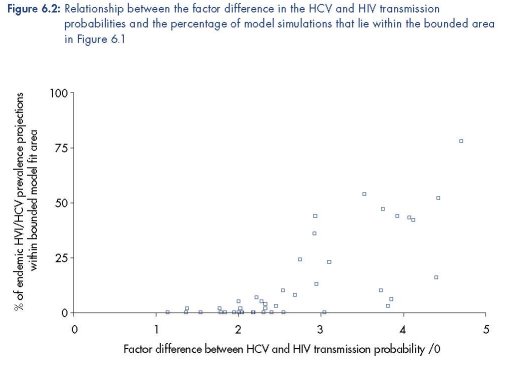
The percentage of model simulations for each biological parameter set that gave endemic
HIV and HCV prevalence projections within the bounded area in Figure 6.1 varied widely
from 0 % to 78 %. Interestingly, the goodness of fit was largely dependent on the factor
difference between the HIV and HCV transmission rate (defined as Ω), with a greater
percentage of simulations lying within the bounded area as Ω increases (Figure 6.2). This
suggests that the HCV transmission probability must be over three, or potentially even four to
five times greater than the HIV transmission probability. Figure 6.2 also shows that one
biological parameter set had a much better goodness of fit than the others, with 78 % of the
model projections within the bounded area compared to 54 % for the next best-fitting
biological parameter set. This biological parameter set was defined as the ‘best fit’ and was
used in the impact analysis.
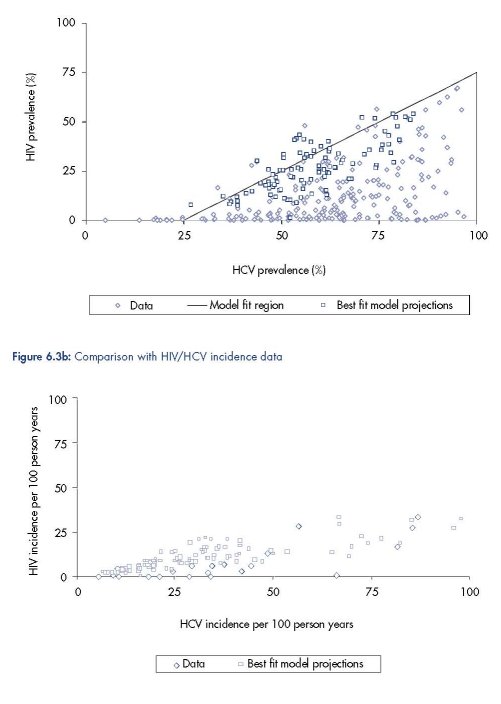
A comparison of available HIV/HCV prevalence and incidence data with the model
projections from the best-fitting biological parameter set is shown in Figures 6.3a and b. They
show that the model mimics the data reasonably well, except for settings with high HCV
prevalence but low HIV prevalence, that is, a low ratio of HIV prevalence to HCV prevalence.
This could be due to the model not incorporating enough heterogeneity in risk behaviour, or
HIV being more compartmentalised in specific IDU networks, or just the fact that we only
included the final time point of the model’s projection of HIV and HCV prevalence for each
epidemic in the comparison. Including all the model projections over time for each epidemic
results in many more lower HIV prevalence projections (result not shown), and the existing
model projections also suggest that decreasing the proportion and/or syringe sharing
frequency of the high-frequency syringe sharers results in a lower HIV prevalence relative to
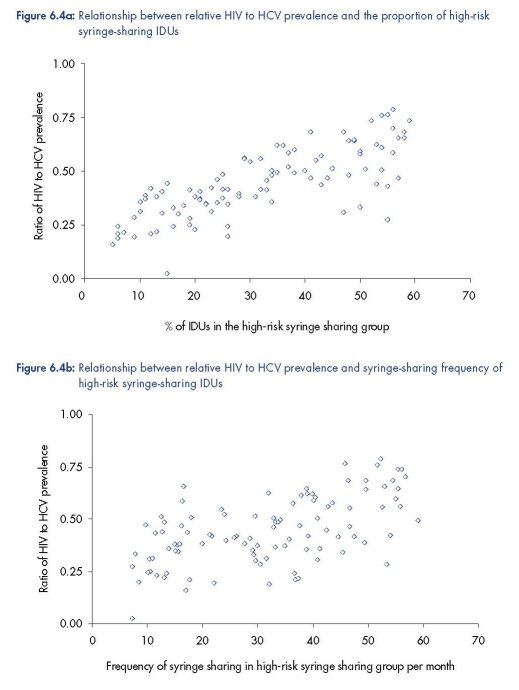
HCV prevalence (Figure 6.4).
Intervention impact projections: overall projections
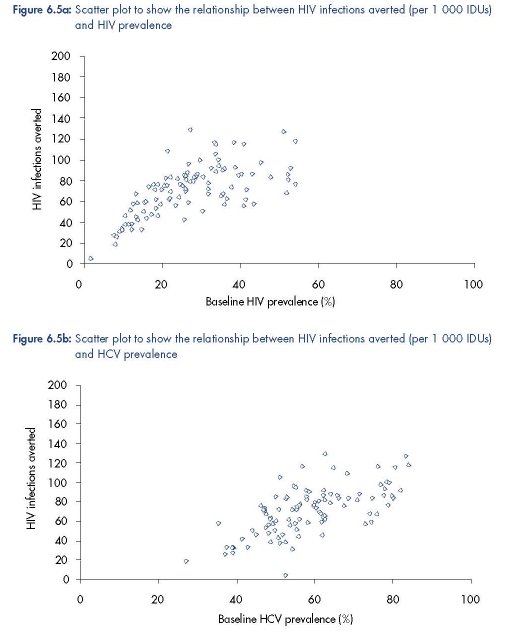
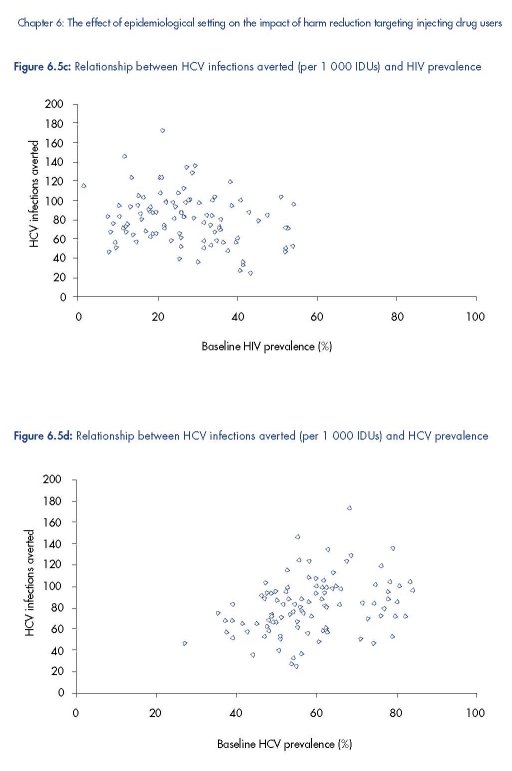
Figure 6.5 shows that the projected HIV and HCV infections averted due to a 50 % reduction
in syringe sharing frequency amongst all IDUs is highly variable, although a similar range of
impact projections are obtained for each infection. The number of HIV infections averted is
positively correlated with baseline HIV and HCV prevalence and the number of HCV
infections averted is negatively correlated with baseline HIV prevalence, but positively
correlated with baseline HCV prevalence. These correlations are maintained when the partial
correlation coefficients are estimated (see Table 6.2), while controlling for the prevalence of
the other infection, with the corresponding linear regression models explaining 50–60 %
(R2=0.5–0.6) of the variance in the model projections.
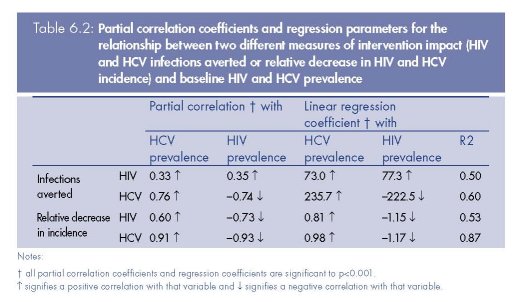
Similar associations are seen for the relative decrease in HIV or HCV incidence due to the
intervention, with both being positively correlated with baseline HCV prevalence and
negatively correlated with baseline HIV prevalence. However, although the regression model
for the relative decrease in HIV incidence has a similar R2, the regression model for the
relative decrease in HCV incidence is a much better fit, explaining 87 % of the variance. The
box below explains the implications of these results.
Implications of regression coefficients relating intervention impact projections to baseline
HIV and HCV prevalence
For every 10 % increase in baseline HIV prevalence:
• the post-intervention reduction in HIV and HCV incidence decreases by ~12 %;
• the number of HIV infections averted over three years increases by ~8 per 1 000 IDUs
reached (which translates to a 10–14 % increase in the number of HIV infections averted if
the baseline HIV prevalence was 20 %);
• the number of HCV infections averted over three years decreases by ~22 per 1 000 IDUs
(which translates to a 33–50 % decrease if the baseline HCV prevalence was 50 %).
For every 10 % increase in baseline HCV prevalence:
• the post-intervention reduction in HIV incidence increases by 8 % and that of HCV incidence
by 10 %;
• the number of HIV infections averted over three years increases by ~7 per 1 000 IDUs
reached (which translates to a 10–14 % increase in the number of HIV infections averted if
HIV prevalence was 20 %);
• The number of HCV infections averted increases by about ~24 per 1 000 IDUs (which
translates to a 33–50 % increase if the baseline HCV prevalence was 50 %.
The existence of these associations is due to the relative HIV prevalence (compared to
HCV) being a measure of the proportion and syringe sharing frequency of the high-risk
IDUs (Figure 6.4). To attain high HIV prevalence, a larger proportion of IDUs who
frequently share syringes is necessary, and so it becomes harder to avert HCV infections
and reduce the HIV and HCV incidence because IDUs become re-infected frequently. This
effect is not observed in the HIV infections averted because the force of infection (the
rate at which susceptible individuals become infected) for HIV is much lower than that for
HCV, with the reduced impact on HIV incidence being offset by the higher baseline HIV
incidence.
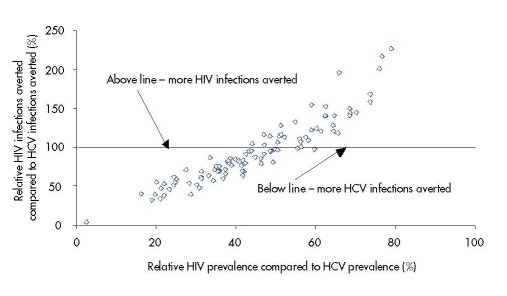
Figure 6.6 suggests that the relative HIV prevalence could be a good predictor of the relative
number of HIV vs. HCV infections averted by an intervention. For example, if HIV prevalence
is 25 % of HCV prevalence then the model suggests that 40–60 % fewer HIV than HCV
infections will be averted, whereas if HIV prevalence is 75 % of HCV prevalence then >50 %
more HIV than HCV infections will be averted.
These results occur because the relative HIV to HCV prevalence in a stable epidemic is a
measure of relative proportion and level of risk behaviour of the high-risk IDUs. For a low
relative HIV prevalence to HCV prevalence, the IDU population must have a smaller and/or
‘less risky’ high-risk group with lower HIV incidence, and so fewer HIV cases but more HCV
cases are averted because fewer of the HCV incident infections are amongst very high-risk
IDUs with a high re-infection rate. For a high relative HIV prevalence, the IDU population
must have a larger and/or ‘more risky’ high-risk group with higher HIV incidence, and so
more HIV cases but fewer HCV cases are averted because more HCV incident infections are
amongst the high-risk IDUs that frequently get re-infected.
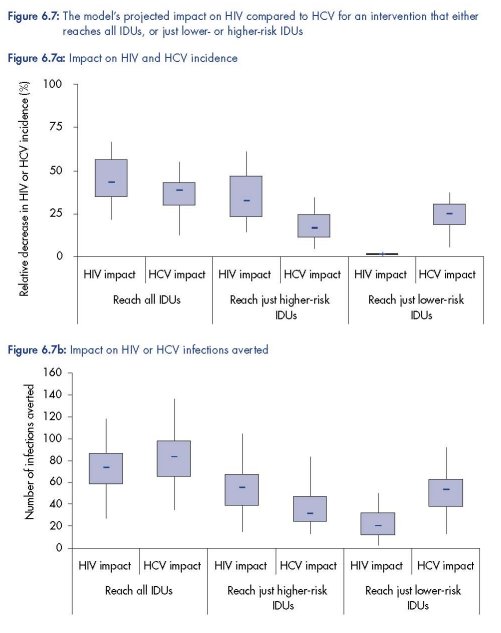
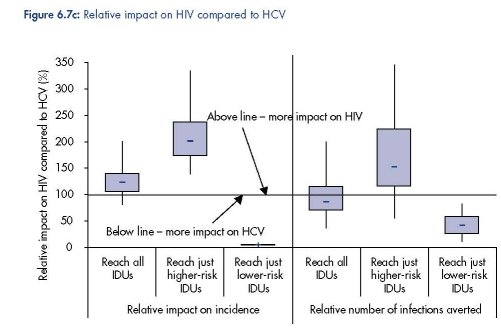
Intervention impact projections: effect of targeting IDUs
Figure 6.7 shows that the targeting of an intervention among IDUs to only those IDUs that
have a low- or high-frequency of syringe sharing could have substantial implications for the
impact of an intervention. If the intervention just reaches the higher-risk IDUs then a greater
impact on HIV transmission can be expected, whereas if the intervention just reaches the
lower-risk IDUs then a much greater impact on HCV transmission should be expected.
Indeed, little to no decrease in HIV incidence should be expected in this latter case because
most HIV transmission occurs amongst the higher-risk IDUs.
Discussion
This study extensively fit a mathematical model to observed trends between HIV and HCV
prevalence in different IDU populations in Europe and beyond. Through fitting the model to a
wide range of different joint HIV and HCV epidemics, we were able to explore why different
HIV and HCV prevalence trends occur in different settings, project how the impact of an
intervention targeting IDUs may vary for different epidemiological settings, and how the
impact of the intervention on HCV transmission may compare to the impact on HIV
transmission. In addition, as a by-product of the fitting process, estimates for the relative
transmissibility of HCV relative to HIV were also produced.
This model analysis should be seen as a significant improvement upon previous modelling
analyses because of the effort made to ensure that the model mimics a wide range of
observed HIV/HCV epidemics. Indeed, through undertaking this novel method of model
fitting, an analysis of the different best-fitting model simulations suggest that heterogeneity in
IDU syringe sharing risk behaviour could be a major determinant for the wide range of HIV
and HCV epidemics that occur in IDU populations in different settings, including Europe. If
this is the case, then the ratio of HIV to HCV prevalence in a stable epidemic setting could be
used as a proxy indicator of the heterogeneity in injecting risk behaviour in that IDU
population.
Key messages of this research
1. Heterogeneity in IDU risk behaviour could be a major determinant for the wide range of
HIV and HCV epidemics that occur in IDU populations in different settings.
2. Model projections suggest that greater intervention impact (more infections averted and
greater decrease in incidence) should be expected in higher HCV prevalence settings and
less impact in higher HIV prevalence settings, except for the number of HIV infections
averted, which increases with HIV prevalence.
3. An intervention will generally result in greater impact on an HIV epidemic than on an HCV
epidemic, in terms of either infections averted or relative decreases in incidence. However,
when the intervention reaches only lower-risk IDUs, it has only little impact on the HIV
epidemic and greater impact on the HCV epidemic.
4. The ratio of HIV to HCV prevalence in a stable epidemic setting could be used as a proxy
indicator of the heterogeneity in injecting risk behaviour in an IDU population, and the
relative number of HIV and HCV infections that would be averted by an intervention.
Relative impact of intervention in different epidemiological settings
As would be expected, our results show that the number of infections averted by a specific
intervention will be highly dependent on the characteristics of the epidemic occurring in a
setting. However, our results also highlight that the relative reduction in HIV or HCV incidence
attained by an intervention can vary by six fold depending on the type of epidemic
occurring. Importantly, the projections imply that the number of infections averted and the
associated relative decrease in incidence are both strongly related to the HIV and HCV
prevalence in a setting. Generally, greater impact (more infections averted and greater
decrease in incidence) is achieved in higher HCV prevalence settings and less impact is
achieved in higher HIV prevalence settings, except for the number of HIV infections averted,
which increases with HIV prevalence.
These results suggest that the highest impact in terms of reducing HIV/HCV incidence and
averting HCV infections should be expected at very high HCV prevalence (>70 %) but low
HIV prevalence (<10 %), such as has been recorded in some settings in Belgium, Greece,
Ireland or Italy. In contrast, the greatest impact in terms of HIV infections averted occurs at
high HIV prevalence (>50 %) as occurs in some settings in Ukraine or Belarus (Vickerman et
al., 2006b; Vickerman and Watts, 2002). These results highlight that policymakers should not
expect interventions to result in the same impact on incidence, or in the same number of
infections averted when initiated in different settings. Indeed, an intervention’s costeffectiveness
ratio will vary widely within and between countries, and different coverage
levels and reductions in syringe sharing will be required to achieve specific reductions in
disease incidence in different settings. This analysis can help in producing these targets and
in estimating the relative cost-effectiveness of specific interventions in different settings.
Interestingly, if HIV and HCV infections are considered in a similar light in public health
terms, as could be the case in many European settings, then the projections have
important implications for how the impact and cost-effectiveness of an intervention is
estimated. For example, although less is achieved in reducing HIV/HCV incidence and
fewer HCV infections are averted in high HIV prevalence settings, interventions can still
have a favourable cost-effectiveness ratio due to many HIV infections being averted.
Alternatively, an intervention undertaken in a high HCV but low HIV prevalence setting, as
occurs in many European regions, may not be seen as cost-effective if only the cost per
HIV infection averted is considered. However, in these settings many more HCV infections
will be averted and the intervention is likely to have a large effect on the transmission of
both infections. To explore this further, quality-adjusted life year (QALY) weights and HIV
and HCV health care costs could be incorporated to give relative weights to the benefits
of a HIV or HCV infection averted, so that the cost-effectiveness of different interventions
can be compared in different epidemiological settings depending on the level of care
provided in that country.
HIV and HCV impact of targeting low- or high-risk IDUs
When the HIV and HCV impact projections are compared further, they suggest that an
intervention will generally result in greater decreases in HIV incidence than HCV incidence.
This is especially true if the intervention mainly reaches higher-risk IDUs, and highlights that
greater coverage and reductions in risk behaviour will frequently be required by interventions
to achieve a comparable impact on HCV incidence as well as HIV incidence. This trend also
occurs, and is more pronounced, when we look at the relative decrease in HIV or HCV
prevalence (results not shown). This has important implications for harm reduction
interventions because it suggests that much more needs to be done if the objective of the
intervention is a decrease in HCV transmission.
Limitations of analysis
The analysis was limited in a number of ways. First, it used a limited number of biological
parameter sets from a previous analysis as the basis for the fitting process. This may have
limited the number of model fits that we found and so there may be more uncertainty around
our impact projections. The model was only fit against HIV and HCV prevalence trends. A
different parameter set may have produced a better fit to other ecological data, such as
trends in HIV/HCV co-infection. Behavioural data was not used in the fitting process, and so
it was difficult to determine whether the behavioural parameters used would produce similar
epidemics in real life. In addition, the transmission of HIV through sexual contact was not
incorporated in the model. Although HIV transmission through injecting risk behaviours is
likely to dominate in most situations, sexual transmission could play a substantial role in
settings where the HIV prevalence is already high but injecting risk behaviours have
decreased in recent years. This could affect the relationship not only between HIV and HCV
prevalence, but also, and most likely, between HIV incidence and HIV prevalence or HIV
incidence and HCV incidence.
Only one intervention was considered in the impact analysis and so it is impossible to
determine how the results would vary for other interventions. The impact analysis also assumed
the HIV and HCV epidemics were in a stable state. Importantly, epidemics in the exponential or
early epidemic stage would have resulted in different impact projections. Lastly, the model was
deterministic and did not incorporate the effects on transmission of different syringe sharing
behaviours within different risk networks. These more complex syringe sharing structures may
affect the prevalence of HIV and HCV in different ways, possibly restricting HIV in small IDU
sub-networks, and so could explain the reason why the current model structure was not able to
predict very low HIV prevalences with high HCV prevalences. The effect of these complexities
on our projections will be explored in future analyses involving more complex models.
Concluding remarks
This analysis used a novel technique to fit a joint HIV/HCV model to HIV and HCV prevalence
data from diverse epidemic types, many of which were from European settings. This enabled
the model to explore the impact of interventions in different epidemic settings, with the results
suggesting that HIV and HCV prevalence could be used as predictors of intervention impact
in different settings, including Europe. However, the study should be seen as preliminary
because a limited number of ‘biological’ parameter sets (HIV and HCV transmission
probabilities and natural history parameters) were used in the fitting and the model was only
fit to HIV/HCV prevalence trends. In addition, the analysis did not explore the impact of
interventions such as opioid substitution treatment (OST), HCV and/or HIV antiviral
treatments, and/or combined interventions, which may be essential for resulting in large
reductions in HIV/HCV incidence in some settings. This has recently been highlighted by a
study looking at the long-term impact of intervention activities in Amsterdam (van den Berg et
al., 2007), and will be the focus of future work where sufficient attention can be given to
subtleties such as how increasing the coverage of OST may increase the coverage of syringe
distribution to IDUs on and off OST, and how the behaviour of IDUs may revert after leaving
OST. In addition, previous analyses have suggested that reducing the number of people that
an IDU shares needles/syringes with and the time that IDUs are initially reached by harm
reduction interventions are both important for determining the impact of a harm reduction
intervention. These will also be explored further in future analyses.
Acknowledgements
Peter Vickerman designed the study, developed the model, undertook all analyses and wrote the
first draft of the chapter. Matthew Hickman contributed to the study concept, helped with
interpreting the results, and contributed to writing the manuscript. Tim Rhodes and V. Anna
Gyarmathy helped edit the chapter. The views expressed are those of the authors and cannot be
taken to reflect the official opinions of the London School of Hygiene and Tropical Medicine.
Financial support was provided from a Medical Research Council New Investigators award (held
by P.V.) and the European Monitoring Centre for Drugs and Drug Addiction. Lastly, grateful thanks
goes to Lucas Wiessing and all contributors to the EMCDDA HIV and HCV prevalence database,
which was highly useful for providing data for the meta-analysis used in this model analysis.
References
Aitken, C. K., Tracy, S. L., Revill, P., et al. (2008), ‘Consecutive infections and clearances of different hepatitis C
virus genotypes in an injecting drug user’, Journal of Clinical Virology 41, pp. 293–6.
Baggaley, R. F., Boily, M. C., White, R. G., and Alary, M. (2006), ‘Risk of HIV-1 transmission for parenteral
exposure and blood transfusion: a systematic review and meta-analysis’, AIDS 20, pp. 805–12.
Blower, S. M., Hartel, D., Dowlatabadi, H., Anderson, R. M. and May, R. M. (1991), ‘Drugs, sex and HIV: a
mathematical model for New York City’, Philosophical Transactions of the Royal Society B: Biological Sciences 331,
pp. 171–87.
Bonacini, M., Lin, H. J. and Hollinger, F. B. (2001), ‘Effect of coexisting HIV-1 infection on the diagnosis and
evaluation of hepatitis C virus’, Journal of Acquired Immune Deficiency Syndromes 26, pp. 340–4.
Cox, A. L., Netski, D. M., Mosbruger, T., et al. (2005), ‘Prospective evaluation of community-acquired acutephase
hepatitis C virus infection’, Clinical Infectious Diseases 40, pp. 951–8.
Craine, N., Hickman, M., Parry, J. V., et al. (2009), ‘Incidence of hepatitis C in drug injectors: the role of
homelessness, opiate substitution treatment, equipment sharing, and community size’, Epidemiology and Infection
137 (9), pp. 1255–65.
Currie, S. L., Ryan, J. C., Tracy, D., et al. (2008), ‘A prospective study to examine persistent HCV reinfection in
injection drug users who have previously cleared the virus’, Drug and Alcohol Dependence 93, pp. 148–54.
Daar, E. S., Lynn, H., Donfield, S., et al. (2001), ‘Relation between HIV-1 and hepatitis C viral load in patients
with hemophilia’, Journal of Acquired Immune Deficiency Syndromes 26, pp. 466–72.
Dalgard, O. (2005), ‘Follow-up studies of treatment for hepatitis C virus infection among injection drug users’,
Clinical Infectious Diseases 40 (Supplement 5), pp. S336–S338.
Danis, K., Doherty, L., McCartney, M., McCarrol, J. and Kennedy, H. (2007), ‘Hepatitis and HIV in Northern
Ireland prisons: a cross-sectional study’, Eurosurveillance 12, pp. 9–12.
De Carli, G., Puro, V. and Ippolito, G. (2003), ‘Risk of hepatitis C virus transmission following percutaneous
exposure in healthcare workers’, Infection 31 (Supplement 2), pp. 22–7.
Des Jarlais, D. C., Perlis, T., Arasteh, K., et al. (2005), ‘Reductions in hepatitis C virus and HIV infections among
injecting drug users in New York City, 1990–2001’, AIDS 19 (Supplement 3), pp. S20–S25.
EMCDDA (2008), Annual report 2008: the state of the drugs problem in the European Union, European Monitoring
Centre for Drugs and Drug Addiction, Lisbon. Available at http://www.emcdda.europa.eu/publications/annualreport/
2008.
Fishbein, D. A., Lo, Y., Netski, D., Thomas, D. L. and Klein, R. S. (2006), ‘Predictors of hepatitis C virus RNA levels
in a prospective cohort study of drug users’, Journal of Acquired Immune Deficiency Syndromes 41, pp. 471–6.
Foss, A. M., Watts, C. H., Vickerman, P., et al. (2007), ‘Could the CARE-SHAKTI intervention for injecting drug
users be maintaining the low HIV prevalence in Dhaka, Bangladesh?’ Addiction 102, pp. 114–25.
Gibson, D. R., Flynn, N. M. and McCarthy, J. J. (1999), ‘Effectiveness of methadone treatment in reducing HIV
risk behavior and HIV seroconversion among injecting drug users’, Aids 13, pp. 1807–18.
Gibson, D. R., Flynn, N. and Perales, D. (2001), ‘Effectiveness of syringe exchange programs in reducing HIV risk
behavior and HIV seroconversion among injecting drug users’, AIDS 15, pp. 1329–41.
Goldberg, D., Burns, S., Taylor, A., et al. (2001), ‘Trends in HCV prevalence among injecting drug users in
Glasgow and Edinburgh during the era of needle/syringe exchange’, Scandinavian Journal of Infectious Diseases
33, pp. 457–61.
Grebely, J., Conway, B., Raffa, J. D., et al. (2006), ‘Hepatitis C virus reinfection in injection drug users’,
Hepatology 44, pp. 1139–45.
Grebely, J., Genoway, K., Khara, M., et al. (2007), ‘Treatment uptake and outcomes among current and former
injection drug users receiving directly observed therapy within a multidisciplinary group model for the treatment
of hepatitis C virus infection’, International Journal of Drug Policy 18, pp. 437–43.
Grebely, J., Genoway, K. A., Raffa, J. D., et al. (2008), ‘Barriers associated with the treatment of hepatitis C virus
infection among illicit drug users’, Drug and Alcohol Dependence 93, pp. 141–7.
Grover, G. and Shivraj, S. O. (2004), ‘Survival pattern of reported HIV infected individuals in the city of Delhi
(India)’, Journal of Communicable Diseases 36, pp. 83–92.
Health Protection Agency (2004), Shooting up: infections among injecting drug users in the United Kingdom 2003,
Health Protection Agency, London.
Health Protection Agency (2008), Shooting up: infections among injecting drug users in the United Kingdom 2007,
Health Protection Agency, London.
Hutchinson, S., Bird, S., Taylor, A. and Goldberg, D. (2006), ‘Modelling the spread of hepatitis C virus infection
among injecting drug users in Glasgow: implications for prevention’, International Journal of Drug Policy 17, pp.
211–21.
Kapadia, F., Vlahov, D., Des Jarlais, D. C., et al. (2002), ‘Does bleach disinfection of syringes protect against
hepatitis C infection among young adult injection drug users?’ Epidemiology 13, pp. 738–41.
Kaplan, E. H. and Heimer, R. (1994), ‘A circulation theory of needle exchange [editorial]’, AIDS 8, pp. 567–74.
Kretzschmar, M. and Wiessing, L. G. (1998), ‘Modelling the spread of HIV in social networks of injecting drug
users’, AIDS 12, pp. 801–11.
Kretzschmar, M. and Wiessing, L. (2004), ‘Modelling the transmission of hepatitis C in injecting drug users’, in
Jager, J. C., Limburg, W., Kretzschmar, M., Postma, M. J. and Wiessing, L. (eds), Hepatitis C and injecting drug
use: impact, costs and policy options, European Monitoring Centre for Drugs and Drug Addiction, Lisbon.
Kretzschmar, M. and Wiessing, L. (2008), ‘New challenges for mathematical and statistical modeling of HIV and
hepatitis C virus in injecting drug users’, Aids 22, pp. 1527–37.
Kumarasamy, N., Solomon, S., Flanigan, T. P., et al. (2003), ‘Natural history of human immunodeficiency virus
disease in southern India’, Clinical Infectious Diseases 36, pp. 79–85.
Kwon, J. A., Iversen, J., Maher, L., Law, M. G. and Wilson, D. P. (2009), ‘The impact of needle and syringe
programs on HIV and HCV transmissions in injecting drug users in Australia: a model-based analysis’, Journal of
Acquired Immune Deficiency Syndromes 51, pp. 462–9.
Larghi, A., Zuin, M., Crosignani, A., et al. (2002), ‘Outcome of an outbreak of acute hepatitis C among healthy
volunteers participating in pharmacokinetics studies’, Hepatology 36, pp. 993–1000.
Limburg, W. (2004), Natural history, treatment and prevention of hepatitis C in injecting drug users: an overview, in
Jager, J. C., Limburg, W., Kretzschmar, M., Postma, M. J. and Wiessing, L. (eds), Hepatitis C and injecting drug
use: impact, costs and policy options, European Monitoring Centre for Drugs and Drug Addiction, Lisbon.
Mehta, S. H., Cox, A., Hoover, D. R., et al. (2002), ‘Protection against persistence of hepatitis C’, Lancet 359, pp.
1478–83.
Micallef, J. M., Kaldor, J. and Dore, G. J. (2006), ‘Spontaneous viral clearance following acute hepatitis C
infection: a systematic review of longitudinal studies’, Journal of Viral Hepatitis 13, pp. 34–41.
Micallef, J. M., Macdonald, V., Jauncey, M., et al. (2007), ‘High incidence of hepatitis C virus reinfection within
a cohort of injecting drug users’, Journal of Viral Hepatitis 14, pp. 413–18.
Muga, R., Langohr, K., Tor, J., et al. (2007), ‘Survival of HIV-infected injection drug users (IDUs) in the highly
active antiretroviral therapy era, relative to sex- and age-specific survival of HIV-uninfected IDUs’, Clinical
Infectious Diseases 45, pp. 370–6.
Murray, J. M., Law, M. G., Gao, Z. and Kaldor, J. M. (2003), ‘The impact of behavioural changes on the
prevalence of HIV and hepatitus C among injecting drug users’, International Journal of Epidemiology 32, pp.
708–14.
Pappalardo, B. L. (2003), ‘Influence of maternal human immunodeficiency virus (HIV) co-infection on vertical
transmission of hepatitis C virus (HCV): a meta-analysis’, International Journal of Epidemiology 32, pp. 727–34.
Pilcher, C. D., Price, M. A., Hoffman, I. F., et al. (2004a), ‘Frequent detection of acute primary HIV infection in
men in Malawi’, AIDS 18, pp. 517–24.
Pilcher, C. D., Tien, H. C., Eron, J. J., Jr., et al. (2004b), ‘Brief but efficient: acute HIV infection and the sexual
transmission of HIV’, Journal of Infectious Diseases 189, pp. 1785–92.
Pollack, H. A. (2001), ‘Cost-effectiveness of harm reduction in preventing hepatitis C among injection drug users’,
Medical Decision Making 21, pp. 357–67.
Quinn, T. C., Wawer, M. J., Sewankambo, N., et al. (2000), ‘Viral load and heterosexual transmission of human
immunodeficiency virus type 1. Rakai Project Study Group’, New England Journal of Medicine 342, pp. 921–9.
Thomas, D. L., Astemborski, J., Rai, R. M., et al. (2000a), ‘The natural history of hepatitis C virus infection: host,
viral, and environmental factors’, Jama 284, pp. 450–6.
Thomas, D. L., Astemborski, J., Vlahov, D., et al. (2000b), ‘Determinants of the quantity of hepatitis C virus RNA’,
Journal of Infectious Diseases 181, pp. 844–51.
Thomas, D. L., Rich, J. D., Schuman, P., et al. (2001), ‘Multicenter evaluation of hepatitis C RNA levels among
female injection drug users’, Journal of Infectious Diseases 183, pp. 973–6.
UNAIDS (2004a), AIDS epidemic update, UNAIDS, Geneva, pp. 1–21.
UNAIDS (2004b), Report on the global HIV/AIDS epidemic, UNAIDS, Geneva, pp. 1–225.
van den Berg, C., Smit, C., Van Brussel, G., Coutinho, R. A. and Prins, M. (2007), ‘Full participation in harm
reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus:
evidence from the Amsterdam cohort studies among drug users’, Addiction 102, pp. 1454–62.
Vickerman, P. and Watts, C. H. (2002), ‘The impact of an HIV prevention intervention for injecting drug users in
Svetlogorsk, Belarus: model predictions’, International Journal of Drug Policy 13, pp. 149–64.
Vickerman, P., Hickman, M., Rhodes, T. and Watts, C. H. (2006a), ‘Model projections on the required coverage
of syringe distribution to prevent HIV epidemics among injecting drug users’, Journal of Acquired Immune
Deficiency Syndromes 42, pp. 355–61.
Vickerman, P., Kumaranayake, L., Balakireva, O., et al. (2006b), ‘The cost-effectiveness of expanding harm
reduction activities for injecting drug users in Odessa, Ukraine’, Sexually Transmitted Diseases 33 (Supplement),
pp. S89–S102.
Vickerman, P., Hickman, M. and Judd, A. (2007), ‘Modelling the impact of hepatitis C transmission of reducing
syringe sharing: London case study’, International Journal of Epidemiology 36, pp. 396–405.
Vickerman, P., Hickman, M., May, M., Kretzschmar, M. and Wiessing, L. (2009a), ‘Can HCV prevalence be used
as a measure of injection-related HIV-risk in populations of injecting drug users? An ecological analysis’,
Addiction 105, pp. 311–18.
Vickerman, P., Platt, L. and Hawkes, S. (2009b), ‘Modelling the transmission of HIV and HCV amongst injecting
drug users in Rawalpindi, a low HCV prevalence setting in Pakistan’, Sexually Transmitted Infections 85
(Supplement 2), pp. ii23–30.
Wawer, M. J., Gray, R. H., Sewankambo, N. K., et al. (2005), ‘Rates of HIV-1 transmission per coital act, by
stage of HIV-1 infection, in Rakai, Uganda’, Journal of Infectious Diseases 191, pp. 1403–09.
Westh, H., Worm, A. M., Jensen, B. L., et al. (1993), ‘Hepatitis C virus antibodies in homosexual men and
intravenous drug users in Denmark’, Infection 21, pp. 115–17.
WHO (World Health Organization) (2000a), Hepatitis C fact sheet, WHO, Geneva. Available at http://www.
who.int/mediacentre/factsheets/fs164/en/.
WHO (2000b), WHO drug injecting study — phase II operations manual version 4, des Jarlais D., Friedman, S.
and Perlis, T. (eds), WHO, Geneva, 2000. Available at http://www.who.int/substance_abuse/activities/en/
WHODrugInjectionStudyOperationsManual.pdf (accessed 1 October 2008).
Wiessing, L. and Nardone, A. (2006), ‘Ongoing HIV and viral hepatitis infections in IDUs across the EU, 2001–
2005’, Eurosurveillance 11, pp. E061123 2.
Yazdanpanah, Y., De Carli, G., Migueres, B., et al. (2005), ‘Risk factors for hepatitis C virus transmission to
health care workers after occupational exposure: a European case-control study’, Clinical Infectious Diseases 41,
pp. 1423–30.
| < Prev | Next > |
|---|












