Chapter 5 Harm reduction among injecting drug users — evidence of effectiveness
| Reports - EMCDDA Harm Reduction |
Drug Abuse
Chapter 5 Harm reduction among injecting drug users — evidence of effectiveness
Jo Kimber, Norah Palmateer, Sharon Hutchinson, Matthew Hickman, David Goldberg and Tim Rhodes
Abstract
This chapter synthesises and evaluates the available direct evidence relating to the impact of needle and syringe programmes (NSPs), opioid substitution treatment (OST), drug consumption rooms (DCRs), and peer naloxone distribution (PND) on HIV/hepatitis C (HCV)
incidence/prevalence, injecting risk behaviour and overdose-related mortality. To achieve this, we conducted a review of reviews; a systematic and explicit method used to identify, select and critically appraise relevant findings from secondary level research (systematic
reviews and/or meta-analyses) into an evidence briefing. In the absence of high-quality reviews, appraisal of the evidence was supplemented with a targeted review of the primary literature. We find that there is sufficient review-level evidence that OST reduces HIV
transmission, while the evidence in support of NSPs reducing HIV transmission is more tentative, and for DCRs currently insufficient. There is tentative evidence that OST has limited effectiveness in reducing HCV transmission, and insufficient evidence to support or discount
NSPs or DCRs’ ability to reduce HCV transmission. There is sufficient review-level evidence that NSPs, OST and DCRs reduce self-reported injecting risk behaviour. There is sufficient review evidence that OST reduces risk of overdose mortality, but insufficient evidence to
support or discount the effect of DCRs or PND on overdose deaths at the community level. Our review shows evidence in support of a variety of harm reduction interventions but highlights an uneven presence of high-quality review evidence. Future evaluation of harm
reduction programmes should prioritise methodologically robust study designs.
Keywords: injecting drug use, review methods, and needle syringe programmes, opioid substitution treatment, drug consumption rooms, peer naloxone distribution.
Introduction
Injecting drug use is a global and regional public health problem, with an estimated 15.9 million injecting drug users (IDUs) worldwide and prevalence rates in European Union (EU) Member States ranging between 0.6 and 15.1 per 1 000 population aged 15–65 years
(EMCDDA, 2009; Mathers et al., 2008). IDUs, especially opiate users, experience excess morbidity and mortality, being approximately 10 times more likely to die compared to their non-IDU peers (Bargagli et al., 2006; Degenhardt et al., 2004; Degenhardt et al., 2006).
The primary causes of IDU-related morbidity and mortality are blood-borne viruses (BBVs) and drug overdose (Degenhardt et al., 2006). The prevention of BBV infections and drug overdose deaths among IDUs in community and custodial settings is a key objective
of the EU drug strategy (European Commission, 2007). Interventions that directly target these harms include: needle and syringe programmes (NSPs) and opioid substitution treatment (OST); supervised drug consumption rooms (DCRs), and peer naloxone
distribution (PND).
NSPs provide sterile needle/syringes and other injecting equipment to IDUs. Delivery is diverse and can include ‘primary’ fixed site, mobile and/or outreach services and ‘secondary’ access via community pharmacies, other health services and/or vending machines (WHO, 2007). NSPs operate across all EU Member States (EMCDDA, 2008; see also Cook et al., 2010).
OST is prescribed to dependent users to diminish the use and effects of illicit opiates. Treatment is most efficacious when it is continuous and given at adequate doses (Amato et al., 2005; Faggiano et al., 2003; Ward et al., 1997). Community-based OST is available
across all EU Member States and prison-based OST is officially available in the majority of Member States, although overall accessibility is limited (EMCDDA, 2008; see also Stevens et al., 2010). It is estimated that in 2007 more than 650 000 opioid users received OST in
Europe, and the most commonly prescribed forms are methadone maintenance treatment (MMT) and buprenorphine maintenance treatment (BMT) (EMCDDA, 2008).
DCRs offer a low-threshold environment to use pre-obtained drugs hygienically and to access targeted safer injecting advice and intervention in case of overdose (Kimber et al., 2003; see also Hedrich et al., 2010). DCRs have been operating in Europe for more than 25 years and are available in 59 cities across Germany, Luxembourg, the Netherlands, Norway, Spain and Switzerland (EMCDDA, 2008).
Peer naloxone distribution (PND) or ‘take-home naloxone’ programmes provide the antagonist drug, with training to IDUs and/or carers to improve their capacity for effective intervention at opioid-related overdose (Darke and Hall, 1997). Naloxone is currently available on a take-home basis in Italy (where it is widely dispensed by addiction services), Germany, Spain, Lithuania and Norway (Reitox, 2008). PND pilots have also taken place (Dettmer et al., 2001; McAuley et al., 2009; Strang et al., 2008) and are underway (National Treatment Agency, 2009; Parmar, 2008) in the United Kingdom.
The availability and delivery of harm reduction interventions can be controversial outside of the public health arena and vulnerable to shifts in the political environment at the local, national and international level (Bewley-Taylor, 2002; Broadhead et al., 1999; Small, 2007).
This re-enforces the need for policymakers to have access to up-to-date evidence briefings on the targeted outcomes and effectiveness of the relevant interventions.
In this chapter we synthesise and evaluate the available evidence relating to the impact of NSPs, OST and DCRs on HIV and HCV incidence/prevalence, injecting risk behaviour, and OST, DCRs and PND on overdose-related mortality. We will focus on evidence synthesised in previous evidence reviews, and where necessary supplement with a review of the recently published primary literature.
Methods
Our evaluation of the evidence is based primarily on the ‘review of reviews’, or tertiary level research method (Kelly et al., 2002). This is a systematic and explicit method to identify, select, and critically appraise relevant findings from secondary level research (i.e. systematic
reviews and/or meta-analyses) into an evidence briefing.
We have drawn substantively on our recent review of reviews of harm reduction interventions (Palmateer et al., 2008; Palmateer et al., 2010). Our inclusion criteria were English language systematic reviews, syntheses, or meta-analyses that examined the effectiveness of NSPs, OST and DCRs in relation to HIV and HCV incidence/prevalence and/or injecting risk behaviour outcomes. For this chapter we have updated our previous review of reviews (Palmateer et al., 2008) by searching for any new reviews published between March 2007 and August 2009 and by conducting additional searches for relevant English language systematic reviews, syntheses, or meta-analyses that examined the effectiveness of OST, DCRs and PND in preventing overdose.
Databases searched were: CINAHL, Cochrane Library, EMBASE, IBSS, MEDLINE, and PsycINFO. To identify grey literature and minimise English language publication bias we also searched publications of key international agencies for harm reduction. These included: the
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), the National Institute on Drug Abuse (NIDA), the United States Institute of Medicine, the United Nations Office on Drugs and Crime (UNODC), and the World Health Organization (WHO). All databases
were searched from 1980 to March 2009 except CINAHL, which was searched from 1982 to March 2009. At the screening stage it was apparent that reviews from the 1980s and 1990s had been superseded by more recent reviews and we restricted our appraisal of reviews
published from 2000 onwards.
Abstracts were screened and evaluated by two reviewers to determine if the paper met the inclusion criteria. If there was disagreement regarding the relevance of an abstract the full paper was retrieved for further evaluation. In the event of a lack of consensus a decision was reached by discussing points of disagreement.
Selected reviews were critically appraised using a tool that considers the rigour of the methods used to identify the relevant literature, the appraisal of the primary literature, the quality of the analysis in the case of meta-analysis, and the appropriateness of the conclusions (Kelly et al., 2002; Palmateer et al., 2010).
Reviews rated 1 or 2 were included as high-quality (‘core’) reviews. Reviews rated 3 were retained as ‘supplementary’, not considered to be of sufficient quality to rely on the author’s conclusions but viewed as providing complementary information on the effectiveness of the
interventions.
sufficient; (ii) tentative; (iii) insufficient; or (iv) no evidence from reviews. These classificationsFrom each review, we extracted information on the reviewers’ assessment of the evidence and the number, design and findings of the relevant primary studies. The level of review evidence that supported or discounted the effect of an intervention was classified as: (i) are based on a framework (Table 5.1) that considers the quality of the reviews, the reviewers’ conclusions and the designs/findings of the primary studies (Ellis et al., 2003).

Consistent with an evidence-based medicine approach (Glaziou and Heneghan, 2009; Sackett et al., 1996), study designs considered to provide more ‘robust’ evidence of effect were controlled trials, longitudinal cohort and case-control designs, while ecological, serial
cross-sectional and cross-sectional designs were considered to provide ‘weaker’ evidence of effect. We do not discount the importance of different study designs and data sources, including cross-sectional and qualitative studies, to evaluate the process and impact of public health interventions (Petticrew, 2009), but our aim here is to assess quality of the review-level evidence.
With regard to our interpretation of the reviews’ reported results of primary studies, a ‘positive’ finding refers to an observed reduction in the stated outcome associated with the intervention, a ‘negative’ finding refers to an increase in the outcome associated with the
intervention, and ‘no association’ refers to no statistically significant effect. Where a review reported a finding as positive or negative, it was assumed that the result was statistically significant at the 5 % level even if this was not explicitly stated; where a review reported ‘no
association’ it was assumed that this indicated a non-statistically significant result (Palmateer et al., 2010).
A priori we recognise that no or weak evidence of effectiveness may primarily reflect the quality and/or number of studies available and does not necessarily indicate a lack of intervention effectiveness. We also acknowledge that the history of harm reduction interventions has to a large extent (and necessarily) been driven by community actions and pragmatic public health policies (See also Cook et al., 2010), with some interventions implemented in the absence of high-quality trials or intervention-based research.
Additionally, in the absence of a recent review for an intervention and/or outcome, we supplemented our evaluation of the review-level evidence with a review of subsequently published primary literature using the same search strategy and assessment of evidence quality. Thus we undertook primary literature searches for NSPs and HCV incidence prevalence from 2003, OST and overdose from 2003, DCRs for all outcomes from 2004, and PND and overdose from 2004.
Results
The results of the review of reviews literature search are presented in Figure 5.1. We identified nine (five core and four supplementary) reviews of the effectiveness of NSPs, 11 (three core, six supplementary, two meta-analyses) of OST, four (three supplementary, one meta-analysis) of DCRs, and one supplementary review of PND (Table 5.2).
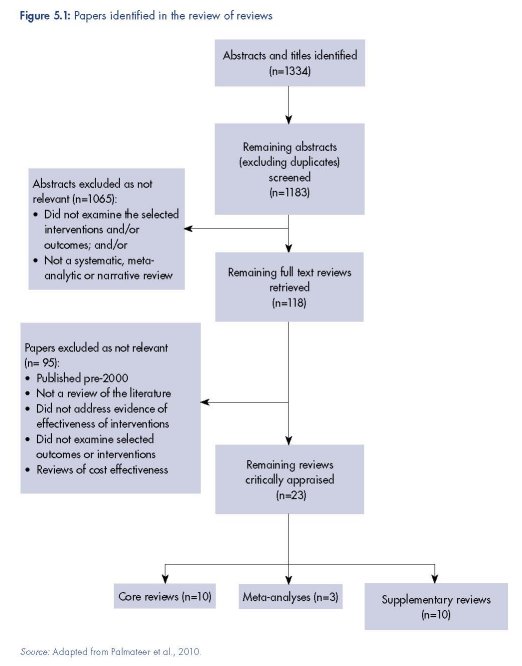
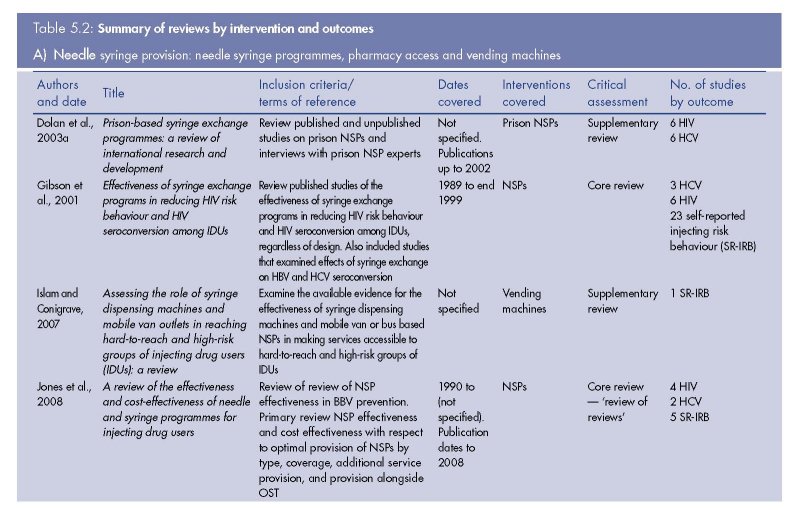
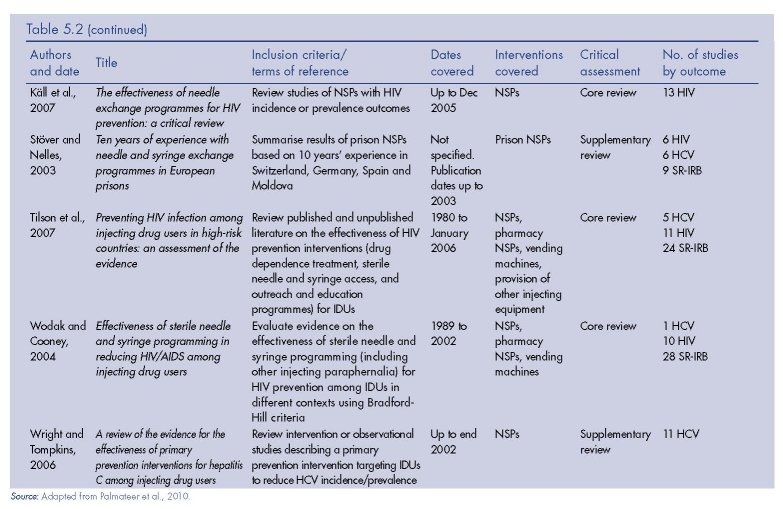
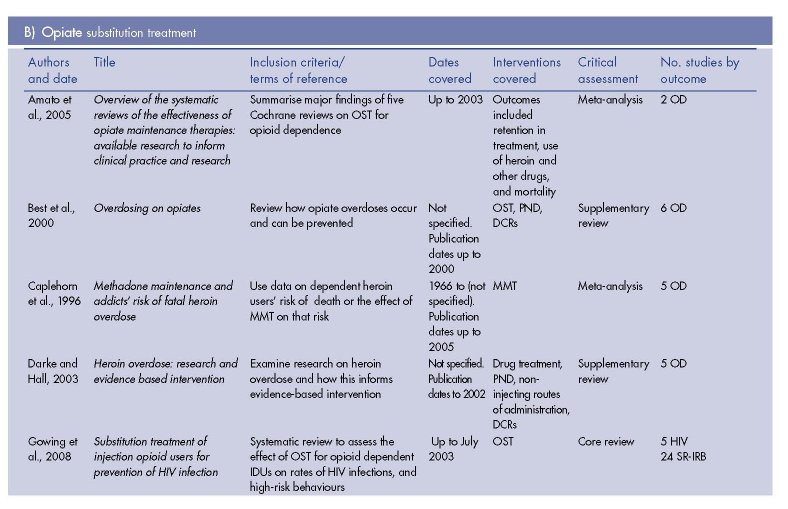
Needle and syringe programmes (1)
Effects on HIV incidence/prevalence
(1) This section on NSPs is largely based on Palmateer et al., 2010.
Evidence of the effects of NSPs on HIV incidence/prevalence was considered in four core reviews (Gibson et al., 2001; Käll et al., 2007; Tilson et al., 2007; Wodak and Cooney, 2004), which included a total of 18 primary studies with HIV incidence or prevalence outcomes.
Tilson et al., 2007, the most recent and rigorous of these reviews, identified 13 relevant studies: four prospective cohort (Bruneau et al., 1997; Mansson et al., 2000; Schechter et al., 1999; Strathdee et al., 1997), two case-control (Patrick et al., 1997; van Ameijden et al., 1992), three ecological (Des Jarlais et al., 2005b; Hurley et al., 1997; MacDonald et al., 2003), and two serial cross-sectional studies (Des Jarlais et al., 2005a; Hammett et al., 2006). Other studies included in their discussion were Des Jarlais et al. (1995) and Coutinho (2005).
Two of the prospective cohort studies (Bruneau et al., 1997; Strathdee et al., 1997) found NSP participation was associated with a higher incidence of HIV seroconversion. Tilson et al., 2007 highlighted that these findings may have been related to several factors, including: restrictive service delivery characteristics; high-risk IDUs being more likely to use the NSP (selection bias); and the availability of clean injecting equipment from other sources (dilution bias).
The authors also refer to four ecological studies demonstrating declining HIV incidence/ prevalence in the context of NSP provision or expansion (Des Jarlais et al., 1995; Des Jarlais et al., 2005b; Hurley et al., 1997; MacDonald et al., 2003). Tilson et al. concluded that: ‘The
evidence of the effectiveness of [NSPs] in reducing HIV prevalence is considered modest, based on the weakness of these study designs’ (2007, p. 149). Their conclusions are consistent with the equivocal results from cohort and case-control studies; this review also undertook the most rigorous evaluation of the primary studies and also considered outcomes related to HIV incidence/prevalence separately from injecting risk behaviour.
Käll and colleagues (2007) identified 13 studies examining NSPs and changes in HIV incidence/prevalence outcomes published to the end of 2005, including 11 studies identified in other reviews and two additional studies (Amundsen et al., 2003; Valente et al., 2001).
With regard to HIV seroincidence, in seven studies there was no reported association with NSPs (Amundsen et al., 2003; MacDonald et al., 2003; Patrick et al., 1997; Schechter et al., 1999; Schoenbaum et al., 1996; Valente et al., 2001; van Ameijden et al., 1992); one study
found a positive effect (Des Jarlais et al., 1996), and one study found a negative effect (Bruneau et al., 1997).
The authors also highlighted three longitudinal studies with a negative baseline association between NSP use and HIV seroprevalence (Bruneau et al., 1997; Millson et al., 2003; Strathdee et al., 1997) and three ecological studies of seroprevalence that found protective effects of NSPs (Health Outcomes International et al., 2002; Hurley et al., 1997), but argued that these studies did not control for probable confounding from differences in the stage of the HIV epidemic relative to the introduction of NSPs. Käll and colleagues concluded that ‘the effectiveness of NEPs to reduce HIV among IDUs is overrated. Errors in categorising studies in favour of NEPs have been made (Wodak and Cooney, 2004; Wodak and Cooney, 2006) and studies claiming positive results have not been adequately scrutinized’ (2007, p. 6).
Wodak and Cooney (2004) did not consider separately the effects of NSPs on HIV transmission versus injecting risk behaviour, and this may have led to the evidence of reduced injecting risk behaviour having a bearing on conclusions drawn with respect to HIV incidence/prevalence: ‘There is compelling evidence that increasing the availability and utilization of sterile injecting equipment by IDU reduces HIV infection substantially’ (p. 28). Of the 38 studies they reviewed, 10 were relevant to HIV (Bruneau et al., 1997; Des Jarlais et al., 1996; Heimer et al., 1993; Hurley et al., 1997; Ljungberg et al., 1991; MacDonald et al., 2003; Monterroso et al., 2000; Patrick et al., 1997; Schechter et al., 1999; Strathdee et al., 1997); five had positive findings (Des Jarlais et al., 1996; Heimer et al., 1993; Hurley et al.,
1997; Ljungberg et al., 1991; MacDonald et al., 2003), two had negative findings (Bruneau et al., 1997; Strathdee et al., 1997), and three did not find an association (Monterroso et al., 2000; Patrick et al., 1997; Schechter et al., 1999). Four of the five positive findings were
generated by studies with weaker designs (Heimer et al., 1993; Hurley et al., 1997; Ljungberg et al., 1991; MacDonald et al., 2003).
Gibson et al. (2001) reviewed studies published up until 1999, all of which were covered in the reviews discussed above. They gave consideration to potential bias in studies with negative results, but not for those with protective findings. They concluded that there is ‘Substantial evidence that syringe exchange programmes are effective in preventing [HIV risk behaviour and] HIV seroconversion among IDU’ (p. 1338). However, as for Wodak and Cooney, their conclusions seemed inconsistent with the HIV studies reviewed: two
cohort studies showed an increased risk of HIV infection associated with NSPs (Bruneau et al., 1997; Strathdee et al., 1997), one (meta-analysis using cohort data) showed a protective effect of NSPs (Des Jarlais et al., 1996), and three (one cohort, two case-control)
showed no association (Patrick et al., 1997; Schechter et al., 1999; van Ameijden et al.,1992).
The United Kingdom National Institute for Clinical Excellence’ review of optimal NSP service delivery (Jones et al., 2008) included a review of reviews component on HIV prevention that evaluated the four reviews considered above. Consistent with our assessment, they
concluded:
There is evidence from two good-quality systematic reviews [Wodak and Cooney, 2004; Gibson et al., 2001] to support the effectiveness of NSPs in reducing HIV infection among IDUs. However, findings from two other systematic reviews [Tilson et al., 2007; Käll et al., 2007], including one good quality review [Tilson et al., 2007], suggest that the evidence may be less convincing.
(Jones et al., 2008, pp. 31–2)
Pharmacy access
Evidence of the effectiveness of pharmacy access to needles/syringes in reducing HIV prevalence was examined in one core review (Wodak and Cooney, 2004), which identified two relevant studies (Hunter et al., 1995; Nelson et al., 1991). A serial crosssectional study observed that declines in HIV prevalence in the United Kingdom coincided with a period of increased access to needles/syringes through pharmacies and NSPs (Hunter et al., 1995). Second, a cross-sectional survey found a lower HIV prevalence in diabetic IDUs, who had ready access to sterile syringes through pharmacies, compared with non-diabetic IDUs (Nelson et al., 1991). They also referred to two studies as evidence of ‘replication of findings’: an ecological study that found pharmacy exchange was a common characteristic of cities that had maintained HIV prevalence rates of less than 5 % over the previous five years (Des Jarlais et al., 1995), and a rapid assessment study that attributed a low HIV infection rate in Georgia in part to the availability of syringes in pharmacies (De Jong et al., 1999).
Wodak and Cooney (2004) concluded that ‘There is reasonable evidence that pharmacy availability of sterile injecting equipment does provide specific benefits in addition to those derived from NSPs’ (p. 29). We note, however, that this is based on a small number of
primary studies with weaker designs.
Vending machines
One core review (Wodak and Cooney, 2004) reported the results of a cross-sectional study of IDUs (Obadia et al., 1999), which found that primary users of vending machines were less likely to be HIV positive, although this was not significant after adjustment in a multivariable model. The authors stated that ‘Access to sterile needles and syringes from community pharmacies and syringe vending machines was shown in all nine studies to be effective in reducing risk behaviour and HIV seroprevalence’ (p. 18). We note, however, that this conclusion is drawn on one study of vending machines with a weak design.
Prison
Two supplementary reviews (Dolan et al., 2003a; Stöver and Nelles, 2003) reported on HIV and HCV incidence from six prison NSP evaluations in Switzerland, Germany and Spain (Jacob and Stöver, 1997; Jacob and Stöver, 2000; Meyeno et al., 2000; Nelles et al., 1997;
Villaneuva, 2002). Based on serological testing in five studies and self-report in one study, no new cases of HIV (or HCV) infections were observed in these prisons during one to two years of follow-up. Both reviews provided limited details about the design and quality of these evaluation studies.
A subsequent German cohort study of prison NSPs and BBV incidence observed four HCV seroconversions among 22 prisoners who were seronegative at baseline during a median 12 months of follow-up (incidence rate 18/100 person years). At least one of these exposures was associated with injecting risk behaviour while in prison (Stark et al., 2006).
Evidence statement for NSPs and HIV incidence/prevalence
Primary NSP
Based on a tentative statement from one core review, supported by consistent evidence from less robust primary studies, we conclude that there is tentative evidence to support the effectiveness of NSPs in reducing HIV incidence/prevalence among IDUs.
Pharmacy access
Despite a tentative statement of effectiveness from a core review, the evidence is based on a small number of primary studies with weak designs. We conclude that there is insufficient review-level evidence to either support or discount the effectiveness of pharmacy access to needles/syringes in reducing HIV prevalence among IDUs.
Vending machines
There is insufficient review evidence to either support or discount the effectiveness of needle/ syringe vending machines in reducing HIV transmission among IDUs.
Prison
Given a lack of evidence from core reviews, and evidence of uncertain quality from supplementary reviews, we conclude that there is insufficient review-level evidence to either support or discount the effectiveness of prison NSPs in reducing HIV transmission among IDUs.
Effects on HCV incidence/prevalence
Evidence of the effects of NSPs on HCV incidence/prevalence was considered in three core reviews of NSPs and HIV (Gibson et al., 2001; Tilson et al., 2007; Wodak and Cooney, 2004) and one supplementary review (Wright and Tompkins, 2006). The core reviews, however, were focused on HIV outcomes and none examined HCV in any detail, covering seven primary studies between them. Wodak and Cooney included one study (Hagan et al., 1995), Tilson et al. identified six (Des Jarlais et al., 2005b; Hagan et al., 1995; Hagan and Thiede, 2000; Mansson et al., 2000; Sarkar et al., 2003; Taylor et al., 2000), and Gibson et al. included three (Hagan et al., 1995; Hagan et al., 1999; Lamden et al., 1998).
Wright and Tompkins (2006) focused exclusively on HCV outcomes, and identified nine additional papers (Goldberg et al., 2001; Goldberg et al., 1998; Hernandez-Aguado et al., 2001; Hutchinson et al., 2002; MacDonald et al., 2000; Patrick et al., 2001; Smyth et al., 1999; Somaini et al., 2000; van Ameijden et al., 1993), although three of these present duplicate data (Goldberg et al., 2001; Goldberg et al., 1998; Hutchinson et al., 2002), and the search only included studies published up until 2002. There were seven primary studies with positive findings, but these mainly involved weaker study designs. The stronger study designs (cohorts) mainly showed either no association or negative findings between NSPs and HCV seroconversion.
Tilson et al. (2007) concluded there was moderate evidence that ‘HIV prevention programmes that include NSPs have less of an impact on HCV transmission than on HIV transmission’ (p. 149). Similarly, in their review of reviews Jones et al. (2008) concluded, ‘There is insufficient evidence from two systematic reviews [Wright and Tompkins, 2006; Tilson et al., 2007] to determine the impact of NSPs on hepatitis C infection in IDUs’ (p. 32).
In our search of the primary literature published since Wright and Tompkins’ we identified three additional cohort studies of HCV incidence and NSP use (Hagan et al., 2004; Roy et al., 2007; van den Berg et al., 2007). Time to HCV seroconversion was not associated
with being an NSP user at baseline after a median of 2.1 years’ follow-up (Hagan et al., 2004) or with NSP use in the preceding six months (Roy et al., 2007). In the Amsterdam Cohort Study among ever IDUs, NSP use alone was not associated with lower risk of HCV
seroconversion but full participation in both NSPs and MMT was associated with a lower risk of HCV infection in ever IDU compared to no participation (van den Berg et al., 2007).
No core or supplementary reviews were identified that examined HCV incidence/prevalence outcomes in relation to pharmacy access or vending machines. For details on prison NSPs and HCV incidence/prevalence see the section ‘Prisons’, p. 128.
Evidence statement for NSPs and HCV incidence/prevalence
Primary NSP
Based on an absence of clear statements from the core reviews, and inconsistent evidence from the primary studies identified in the core reviews and supplementary review, we conclude there is insufficient review-level evidence to either support or discount the effectiveness of NSPs in reducing HCV transmission among IDUs. Evidence from subsequently published longitudinal primary studies suggests no independent association of NSP use on HCV incidence.
Pharmacy access and vending machines
There is no review-level evidence of the effects of pharmacy access to needles/syringes or vending machines on HCV prevalence/incidence among IDUs.
Prison
Given a lack of evidence from core reviews, and evidence of uncertain quality from supplementary reviews, we conclude that there is insufficient review-level evidence to either support or discount the effectiveness of prison NSPs in reducing HCV transmission among
IDUs.
Effects on injecting risk behaviour
The largest body of evidence on the effect of NSPs relates to changes in self-reported injecting risk behaviour. Three core reviews reported on a total of 43 studies, 39 of which showed a positive effect of NSPs in reducing injecting risk behaviour, and 20 of these were
cohort studies.
Tilson et al. (2007) identified 25 studies (Bluthenthal et al., 2000; Cox et al., 2000; Des Jarlais et al., 2000; Gibson et al., 2002; Hagan et al., 1993; Hagan and Thiede, 2000; Hammett et al., 2006; Hart et al., 1989; Hartgers et al., 1992; Huo et al., 2005; Keene et al., 1993; Klee et al., 1991; Longshore et al., 2001; Monterroso et al., 2000; Ouellet et al., 2004; Schoenbaum et al., 1996; van Ameijden and Coutinho, 1998; van Ameijden et al., 1994; van den Hoek et al., 1989; Vazirian et al., 2005; Vertefeuille et al., 2000; Vlahov et al., 1997; Watters, 1994; Wood et al., 2002; Wood et al., 2003), 14 of which were longitudinal cohort studies (Bluthenthal et al., 2000; Cox et al., 2000; Gibson et al., 2002; Hagan and Thiede, 2000; Hart et al., 1989; Huo et al., 2005; Monterroso et al., 2000; Ouellet et al., 2004; Schoenbaum et al., 1996; van Ameijden and Coutinho, 1998; van den Hoek et al., 1989; Vertefeuille et al., 2000; Vlahov et al., 1997; Wood et al., 2002) and demonstrated reductions in self-reported needle sharing (lending or borrowing needles/syringes). They concluded that there was ‘moderate evidence to show that multicomponent HIV prevention programmes that include needle and syringe exchange’ are associated with a reduction in self-reported sharing of needles and syringes’ (Tilson et al., 2007, p. 154).
Wodak and Cooney (2004) identified 28 primary studies of injecting risk behaviour (defined as needle/syringe borrowing, lending, or reuse). Among these studies, there were 24 positive (Bluthenthal et al., 1998; Bluthenthal et al., 2000; Cox et al., 2000; Des Jarlais et al., 1994; Des Jarlais et al., 2000; Donoghoe et al., 1989; Frischer and Elliott, 1993; Gibson et al., 2002; Gleghorn et al., 1998; Guydish et al., 1995; Guydish et al., 1998; Hartgers et al., 1989; Heimer et al., 1998; Keene et al., 1993; Oliver et al., 1994; Paone et al., 1994; Peak et
al., 1995; Power and Nozhkina, 2002; Schoenbaum et al., 1996; Singer et al., 1997; van Ameijden and Coutinho, 1998; van Ameijden et al., 1994; Vlahov et al., 1997; Watters, 1994), one negative (Klee et al., 1991), and three indeterminate (Donoghoe et al., 1992;
Hartgers et al., 1992; Klee and Morris, 1995) results relating to the association between NSPs and injecting risk behaviour. The reviewers did not formulate any conclusions specifically regarding injecting risk behaviour.
The 23 studies identified by Gibson et al. (2001) (Bluthenthal et al., 1998; Broadhead et al., 1999; Des Jarlais et al., 1994; Donoghoe et al., 1989; Donoghoe et al., 1992; Frischer and Elliott, 1993; Guydish et al., 1995; Guydish et al., 1998; Hagan et al., 1994; Hartgers et al.,
1989; Hartgers et al., 1992; Keene et al., 1993; Klee et al., 1991; Klee and Morris, 1995; Oliver et al., 1994; Paone et al., 1994; Peak et al., 1995; Schoenbaum et al., 1996; Singer et al., 1997; van Ameijden and Coutinho, 1998; van Ameijden et al., 1994; Vlahov et al.,
1997; Watters, 1994) were covered in the later core reviews, with the exception of Broadhead et al., 1999, and Hagan et al., 1994. Both studies suggested a protective effect of NSP: Broadhead et al. noted an increase in the reported reuse and sharing of syringes after the closure of an NSP, and Hagan et al. observed a decline in the proportion borrowing used syringes among NSP attendees (pre- vs. post-intervention comparison).
The authors concluded that there is substantial evidence that NSPs are effective in preventing HIV risk behaviour among IDUs.
Pharmacy access
Two core reviews examined evidence of the effects of pharmacy access to needle/syringes and injecting risk behaviour and identified a total of seven studies. Tilson et al. (2007) identified two serial cross-sectional studies that compared injecting risk behaviour before and after liberalisation of the laws permitting syringe sale from pharmacies in New York (Pouget et al., 2005) and Connecticut (Groseclose et al., 1995); both found that reports of syringe sharing among IDUs declined. The authors concluded, ‘A few studies have examined the impact on drug-related HIV risk, and found suggestive evidence of a reduction’ (p. 160). Wodak and Cooney (2004) reported on a further five cross-sectional studies (Caslyn, 1992; Gleghorn et al., 1995; Ingold and Ingold, 1989; Nelson et al., 1991; Richard et al., 2002) and all found pharmacy access was associated with lower levels of injecting risk behaviour.
Vending machines
Two core reviews, Tilson et al. (2007) and Wodak and Cooney (2004), both referred to a pilot study of vending machines in a German prison (Heinemann and Gross, 2001), although their reporting of the study results differs. Wodak and Cooney reported that significant decreases in needle-sharing subsequent to the introduction of the programme were found, whereas Tilson et al. stated that this study showed that IDUs will use vending machines as a source of sterile needles/syringes. Tilson et al. concluded that there was insufficient evidence of the effectiveness of vending machines in reducing HIV risk; the conclusions of Wodak and Cooney are as above, for HIV.
A supplementary review of vending machines (Islam and Conigrave, 2007) identified 37 studies of vending machines that reportedly engaged ‘hard to reach and high risk’ IDUs, but no details were provided on these studies or changes in injecting risk behaviour associated
with access to vending machines.
Prison
A supplementary review (Stöver and Nelles, 2003) reported on nine prison NSP evaluations that had examined injecting risk behaviour. Seven studies found large declines in needle/ syringe sharing or reuse, one study found single cases of sharing and one study found no
change in needle sharing. However, few details were provided on the primary study designs or formal data analysis.
Evidence statement for NSPs and self-reported injecting risk behaviour
Primary NSP
Based on consistent evidence across multiple robust studies, as well as moderate to strong statements of evidence in support of an effect of NSPs on self-reported injecting risk behaviour from two core reviews, there is sufficient review-level evidence to support the effectiveness of NSPs in reducing self-reported injecting risk behaviour among IDUs.
Pharmacy access
Based on less robust studies identified within two core reviews, there is tentative review-level evidence to support the effectiveness of pharmacy access to needles/syringes — in addition to dedicated NSPs — in reducing self-reported injecting risk behaviour among IDUs.
Vending machines
Given conflicting statements of evidence from core reviews based on one primary study with a weak design, there is insufficient review-level evidence to either support or discount the effectiveness of vending machines in reducing injecting risk behaviour among IDUs.
Prison
Despite consistent findings across multiple studies in a supplementary review suggesting reductions in injecting risk behaviour, due to a lack of information on the quality of the studies we conclude that there is insufficient review-level evidence to either support or discount the effectiveness of prison NSPs in reducing self-reported injecting risk behaviour among IDUs.
Opiate substitution treatment (2)
Effects on HIV incidence/prevalence
Evidence of the effects of OST on HIV incidence/prevalence was considered in three core reviews (Gowing et al., 2008; Sorensen and Copeland, 2000; Tilson et al., 2007), which identified eight studies between them (Dolan et al., 2003b; Hartel and Schoenbaum, 1998; Metzger et al., 1993; Moss et al., 1994; Novick et al., 1990; Rhoades et al., 1998; Serpelloni et al., 1994; Williams et al., 1992). These comprised two randomised control trials (RCTs) (Dolan et al., 2003b; Rhoades et al., 1998), four cohort studies (Hartel and Schoenbaum, 1998; Metzger et al., 1993; Moss et al., 1994; Williams et al., 1992), one case-control study (Serpelloni et al., 1994), and one cross-sectional study (Novick et al., 1990). (2) Most of the review-level evidence on the effectiveness of OST relates to MMT, but the findings can be largely taken to refer to OST in general.
Three cohort studies showed the odds of HIV seroconversion were greater for untreated individuals or those with interrupted MMT compared to those who remained continuously in MMT (Metzger et al., 1993; Moss et al., 1994; Williams et al., 1992). A cohort study and case control study showed lower daily dose and more time out of MMT was also associated with higher risk of HIV seroconversion (Hartel and Schoenbaum, 1998; Serpelloni et al., 1994). In an RCT of 50mg versus 80mg MMT no seroconversions occurred in six months of follow-up (Rhoades et al., 1998). A retrospective cohort study found no HIV seroconversions among long-term MMT patients (Novick et al., 1990). An RCT of MMT in prison found no difference in HIV incidence between those in MMT and waitlist controls, although this was in the context of a short period of follow-up and low HIV prevalence (Dolan et al., 2003b).
The conclusions from all three reviews allowed that continuous MMT is associated with lower rates of HIV seroconversion while acknowledging that those who resist treatment or leave treatment may inherently engage in more HIV risk behaviours than those who stay in treatment longer.
Specifically, Gowing et al. (2008), in their Cochrane Review (updated from an earlier version in 2004), concluded, ‘Few data … limit the conclusiveness of any analysis, but these studies consistently indicate lower rates of [HIV] seroconversion associated with substitution
treatment. This suggests that reductions in risk behaviour do translate into actual reduction in cases of HIV infection’ (p. 22); Tilson et al. (2007) concluded that:
Modest evidence from prospective cohort and case-control studies shows that continuous opioid agonist maintenance treatment is associated with protection against HIV seroconversion. This association persists after controlling for many confounders. These studies also show that the risk of HIV seroconversion is inversely related to length of time in treatment. However the possibility of bias in these findings from self selection cannot be ruled out. (Tilson et al., 2007, p. 92)
Finally, Sorensen and Copeland (2000) concluded that:
Four out of the six studies reviewed … provided firm evidence for the protective effect of MMT against HIV seroconversion. These findings are more convincing because they are based on biologically verified outcomes … [but] nearly all the studies are inherently limited by a selfselected treatment sample.
(Sorensen and Copeland, 2000, p. 27)
Prison
One core review of OST (Gowing et al., 2008) and two supplementary reviews of prison OST (Stallwitz and Stöver, 2007; WHO et al., 2007) identified the same RCT described above (Dolan et al., 2003b).
Evidence statement for OST and HIV incidence/prevalence
Based on consistent evidence from three core reviews, there is sufficient review-level evidence to conclude that OST in community settings is effective in reducing HIV seroconversion, especially among those in continuous treatment.
There is insufficient review-level evidence to draw conclusions about the effect of OST on HIV seroconversion in prison settings. Data from one RCT in a jurisdiction with low HIV prevalence found no difference in HIV incidence between those receiving MMT and controls.
Effects on HCV incidence/prevalence
One supplementary review (Wright and Tompkins, 2006) examined evidence of the effects of OST on HCV incidence/prevalence and identified six studies. A cohort and a casecontrol study found a non-significant trend toward lower HCV incidence among those in MMT compared to those not in treatment (Rezza et al., 1996) or those who have left treatment (Thiede et al., 2000). A Dutch cohort study found MMT (in combination with NSPs) was not associated with any decreases in annual HCV incidence over four years (van Ameijden et al., 1993). Three cohort studies did not find any differences in HCV incidence between those in MMT and those not in MMT (Chamot et al., 1992; Crofts et al., 1997; Selvey et al., 1997).
Wright and Tompkins (2006) concluded that, ‘As regards methadone maintenance therapy, whilst it has been successful in reducing the incidence of HIV, the evidence for its effectiveness in reducing HCV incidence is less convincing’ (p. 5).
In our primary literature search we identified five community-based studies of HCV and OST published since Wright and Tompkins’ review. Three cohort studies suggested a positive impact of OST: HCV incidence was lower among those in continuous OST compared with those with interrupted OST (Hallinan et al., 2004); MMT in the past six months was protective against both primary (non-infected IDUs) and secondary (mono-infected IUDs) HIV and HCV infection (Miller et al., 2004); and HCV incidence was similar among those who were not in OST during followup or in OST for up to six months, but was lower amongst those in treatment for 7 to 12 months (Craine et al., 2009). One cohort study found no difference in risk of HCV seroconversion among IDUs recruited from MMT clinics and IDUs recruited from NSPs (Maher et al., 2006) and in the Amsterdam Cohort Study, as described earlier, MMT alone was not associated with lower risk of HCV seroconversion but full participation in both MMT and NSPs was associated with a lower risk of HCV infection (van den Berg et al., 2007).
Prison
Two supplementary reviews of prison OST (Stallwitz and Stöver, 2007; WHO et al., 2007) identified two linked studies (Dolan et al., 2003b; Dolan et al., 2005). There was no difference in HCV incidence between RCT prison MMT and waitlist control groups at fivemonth
follow-up (Dolan et al., 2003b). However, at four-year follow-up, retention in MMT was associated with reduced HCV infection, while short MMT episodes (less than five months) were significantly associated with greater risk of HCV (Dolan et al., 2005).
Evidence statement for OST and HCV incidence/prevalence
Based on consistent evidence showing weak or no association from multiple longitudinal studies within a supplementary review, we conclude that there is tentative review-level evidence of OST having limited impact on HCV transmission. However, taken together with
recently published primary studies, the available evidence suggests OST contributes to a reduction in the risk of HCV seroconversion among those in continuous treatment. There is insufficient review-level evidence to either support or discount the effectiveness of OST with respect to HCV transmission in prison settings. One RCT suggests that retention in MMT from prison to community settings is associated with reduced HCV incidence.
Effects on injecting risk behaviour
Three core reviews examined the effect of OST on injecting risk behaviour. The evidence falls into three broad categories: prevalence and frequency of injection; sharing of injecting equipment; and scores of drug-related risk.
Gowing et al. (2008) identified one RCT (Dolan et al., 2003b) and six cohort studies that reported the prevalence of injecting drug use before and after OST (Camacho et al., 1996; Chatham et al., 1999; Gossop et al., 2000; King et al., 2000; Magura et al., 1991; Teeson et al., 2006); three RCTs (Dolan et al., 2003b; Lott et al., 2006; Strang et al., 2000) and six cohort studies that reported frequency of injection at baseline and followup (Batki et al., 1989; Brooner et al., 1998; Camacho et al., 1996; Chatham et al., 1999; Kwiatkowski and Booth, 2001; Simpson et al., 1995); and two cohort studies that examined both the proportion and frequency of injection (Camacho et al., 1996; Chatham et al., 1999). Tilson et al. identified the same studies except Teeson et al., 2006 and Lott et al., 2006. The studies varied in terms of follow-up periods (range 3 to 12 months) and the measurement of frequency of injecting, but all studies showed statistically significant decreases in injecting risk behaviour from baseline to follow-up (Gowing et al., 2008; Tilson et al., 2007).
Sorensen and Copeland (2000) refer to a further nine studies with data on injection prevalence and frequency: one RCT and four cohort studies of in-treatment samples showed retention in MMT was associated with decreases in injection frequency (Abbott et al., 1998; Ball et al., 1998; Iguchi, 1998; Saxon et al., 1994; Shore et al., 1996); and one cohort and three cross-sectional studies comparing those in treatment with non-treatment samples found MMT associated with fewer injections (Baker et al., 1995; Greenfield et al., 1995; Meandzija
et al., 1994; Stark et al., 1996).
Gowing et al. (2008) identified three RCT and six cohort studies that examined the proportion who reported sharing equipment before and after a period of MMT. Tilson et al. (2007) identified the same studies except Teeson et al. (2006) and Schroeder et al. (2006). Eight out of nine (Camacho et al., 1996; Chatham et al., 1999; Dolan et al., 2003b; Gossop et al., 2000; Grella et al., 1996; Margolin et al., 2003; Schroeder et al., 2006; Teeson et al., 2006) found a significant reduction in sharing between baseline and followup. The ninth study (King et al., 2000), found a non-significant reduction in reported sharing.
Sorensen and Copeland (2000) additionally reported on one RCT and three cohort studies of in-treatment samples that showed that retention in MMT was associated with decreases in sharing of injecting equipment (Camacho et al., 1996; Magura et al., 1998; Rhoades et al., 1998; Saxon et al., 1994) and one cross-sectional study that found no differences in sharing between new treatment entrants and the rest of the sample (Caslyn et al., 1991). One cohort study and four cross-sectional studies comparing those in treatment with non-treatment found MMT was associated with decreased sharing (Caplehorn and Ross, 1995; Greenfield et al., 1995; Klee et al., 1991; Longshore et al., 1993; Stark et al., 1996) and one cross-sectional study found no differences in sharing (Baker et al., 1995).
Gowing et al. (2008) identified four RCTs, one cohort and two cross-sectional studies comparing drug-related HIV risk scores among those in and out of OST (Abbott et al., 1998; Avants et al., 1998; Baker et al., 1995; Chatham et al., 1999; Mark et al., 2006; Marsch et al., 2005; Sees et al., 2000). Tilson et al. (2007) identified the same studies except Mark et al. (2006) and Marsch et al. (2005). Four studies (Abbott et al., 1998; Avants et al., 1998; Chatham et al., 1999; Marsch et al., 2005) found significant decreases in drug-related HIV risk behaviour scores before and after OST. Sees et al. (2000) found no significant difference in mean risk scores between intake and six-month follow-up between MMT and methadone detoxification groups. Finally, Baker et al. (1995) and Mark et al. (2006) compared the drug risk scores for those currently in OST and not in OST and in both studies the mean score was significantly lower for the cohort receiving OST at the time of interview.
The conclusions of all three core reviews allowed that OST was associated with reductions in self-reported prevalence and frequency of injection, sharing of injecting equipment and injecting risk behaviour risk scores. Gowing et al. (2008) concluded:
Substitution treatment is associated with a significant decrease in the proportion of participants reporting injecting drug use and in the frequency of injection … [and] a significant decrease in the sharing of injecting equipment … studies that reported [injecting risk behaviour] scores also showed a significant reduction is risk associated with substitution treatment.
(Gowing et al., 2008, pp. 19–20)
Tilson et al. (2007) concluded:
Moderate to strong evidence from one RCT and a number of observational studies show that patients receiving methadone maintenance treatment report reductions in several drug-related HIV risk behaviours, including frequency of injecting and sharing of injecting equipment. These patients also had lower summary scores of drug-related risk behaviour compared with pre-treatment levels.
(Tilson et al., 2007, p. 89)
Sorensen and Copeland (2000) concluded,
26 out of 28 studies showed positive results in reducing HIV risk behaviours … In this review both longitudinal studies of in-treatment samples and studies comparing treatment patients with other samples found very strong evidence that drug abuse treatment decreases the risk of HIV infection by decreasing needle-use. The evidence is less strong, but still substantial, that drug abuse treatment changes the needle use patterns of participants (e.g. less needle-sharing, more use of sterile needles).
(Sorensen and Copeland, 2000, pp. 27–8)
Prison
Two supplementary reviews examined prison OST and injecting risk behaviour (Stallwitz and Stöver, 2007; WHO et al., 2007). WHO et al. identified seven studies of prison-based OST and injecting risk behaviour (Bayanzadeh et al., undated; Boguña, 1997; Dolan et al., 1996; Dolan et al., 1998; Dolan et al., 2003b; Heimer et al., 2005; Heimer et al., 2006), although some of these studies reported very similar findings from the same data set (i.e. Heimer et al., 2005 and 2006; Dolan et al., 1996 and 1998). Stallwitz and Stöver (2007) also referred to three studies included in WHO et al. (Boguña, 1997; Dolan et al., 1998; Dolan et al., 2003b). Across all studies, opioid-using IDUs who received MMT in prison reported injecting significantly less frequently than those not receiving MMT in prison. WHO et al. concluded: ‘Prison-based OST programmes appear to be effective in reducing the frequency of injecting drug use and associated sharing of injecting equipment, if a sufficient dosage is provided and treatment is provided for longer periods of time’ (p. 9).
Evidence statement for OST and injecting risk behaviour
Based on consistent evidence from multiple robust studies in three core reviews there is sufficient review-level evidence to support the effectiveness of OST in reducing the frequency of injection, the sharing of injecting equipment and injecting risk behaviour scores.
Based on consistent evidence from two supplementary reviews there is tentative evidence to support the effectiveness of prison-based OST in reducing injecting risk behaviour among IDUs in prison by reducing frequency of injection of heroin and other opiate use.
Effects on overdose-related mortality
We identified two meta-analyses (Amato et al., 2005; Caplehorn et al., 1996) and three supplementary narrative reviews that considered OST and overdose-related mortality (Best et al., 2000; Darke and Hall, 2003; Sporer, 2003). Between them they referred to 14 primary studies (Caplehorn et al., 1994; Cushman, 1977; Darke and Ross, 1999; Darke et al., 2000; Davoli et al., 1993; Fugelstad et al., 1995; Gearing and Schweitzer, 1974; Gronbladh et al., 1990; Gunne and Gronbladh, 1981; McGregor et al., 2002; Poser et al., 1995; van Ameijden et al., 1999; Yancovitz et al., 1991; Zador et al., 1996).
Amato et al. (2005) synthesised the results of five systematic reviews of OST effectiveness in treating opioid dependence. However, they were only able to pool data for all cause mortality for MMT versus waiting list/no treatment from two controlled studies (Gunne and Gronbladh, 1981; Yancovitz et al., 1991) and found non-significant trend suggestive of a reduced risk of death in MMT (RR 0.15, CI 0.02-1.0). The authors concluded, ‘Death within the time frames of a clinical trial is a rare event, even in a high risk population like opiate users
… for the statistical power needed to study mortality, big RCTs or long follow-up periods are required’ (p. 325).
Caplehorn et al. (1996) conducted a meta-analysis of mortality in and out of MMT based on the results of the aforementioned RCT (Gunne and Gronbladh, 1981) and five cohort studies (Caplehorn et al., 1994; Cushman, 1977; Gearing and Schweitzer, 1974; Gronbladh et al., 1990; Poser et al., 1995) from Sweden, Germany, Australia and the United States. MMT reduced risk of death by 75 % (relative risk 0.25, CI 0.19-0.33), due almost entirely to decreases in deaths due to overdose. Notably the included results were all from high-dose
programmes. They concluded:
Addicts were one-quarter as likely to die while in methadone maintenance treatment because they were less likely to die from heroin overdose or suicide. These are most probably direct, pharmacological effects of methadone and are likely to be dose-dependent. This conclusion gives strong support to the argument that all heroin addicts should have access to high-dose, long term maintenance treatment.
(Caplehorn et al., 1996, p. 190)
Other studies referred to in the three supplementary reviews (Best et al., 2000; Darke and Hall, 2003; Sporer, 2003) highlight that overall risk of overdose death is reduced significantly while in treatment compared to never being in treatment or after leaving treatment. An Italian case-control study of 4 200 IDUs found the risk of overdose death was over three times higher among those who left MMT compared to those still in treatment, and over seven times higher in the first 12 months after leaving treatment (Davoli et al., 1993). In a Swedish cohort study of 472 HIV-infected IDUs, risk of death by overdose or trauma was reduced by 75 % while in MMT compared to never being in treatment
(Fugelstad et al., 1995).
A trend toward a dose-dependent reduction in the risk of overdose death was also observed in a Dutch cohort study, where the risk of death among those on 55 mg or more was a third of that in patients on lower doses (van Ameijden et al., 1999). Additionally systematic audits of coronial data on heroin-related deaths in Australia have shown that around 98 % of deaths occurred among individuals not enrolled in MMT at the time of their death (Darke and Ross, 1999; Darke et al., 2000; McGregor et al., 2002; Zador et al., 1996).
Conclusions from all three supplementary reviews consistently supported that being in OST is associated with a substantial reduction in the risk of opioid overdose compared to no treatment or after leaving treatment. Best et al. (2000) also highlighted that the risk of overdose death during treatment is greatest during induction and that risk of death out of treatment is greatest immediately after leaving/being dropped from treatment.
We identified four longitudinal primary studies of OST and overdose-related mortality published since 2003, which all found significant reductions in mortality risk during treatment compared to when out of treatment (Brugal et al., 2005; Clausen et al., 2008; Davoli et al., 2007; Degenhardt et al., 2009).
An Italian prospective cohort study of 10 454 heroin users entering treatment found those retained in MMT had a 90 % reduced risk of death compared to those not in treatment (Davoli et al., 2007). Similarly in a Spanish cohort of 5 049 heroin users entering treatment, risk of overdose death was seven times greater for those not in MMT at the time of death (Brugal et al., 2005). A Norwegian prospective data linkage study of mortality among 3 789 heroin users who applied for OST showed risk of overdose death was reduced by 80 % while in treatment compared to OST waiting list or after leaving treatment (Clausen et al., 2008). In an Australian state-wide OST retrospective data linkage study of 42 676 individuals entering treatment over a 20-year period, OST contributed to a 29 % reduction in mortality (mostly due to overdose and trauma) across the entire cohort (Degenhardt et al., 2009).
Finally, an ecological study of access to OST and overdose deaths in France during a rapid scale-up of OST (particularly BMT) suggests that as the number of drug users in OST increased, there was a concurrent rapid decline in the annual number of opioid related overdose deaths (Emmanuelli and Desenclos, 2005).
Prison
WHO et al. (2007) note recent release from prison as a significant risk factor for drug overdose and the importance of drug treatment through-care. They identified one study of prison MMT and post-release mortality (Dolan et al., 2005). In a four-year follow-up of 382 prison-based MMT RCT participants, no deaths occurred while participants were in MMT, but 17 died out of MMT (untreated mortality rate of 2.0 per 100 person-years, 95 % CI, 1.2-3.2). Eight deaths were from drug overdose, four had never received MMT and four had ceased
MMT prior to release from prison.
Evidence statement for OST and overdose
Based on consistent evidence from one meta-analysis and multiple robust studies in supplementary reviews, there is sufficient review-level evidence to support the effectiveness of OST in reducing the risk of opioid overdose death. Recently published high-quality primary
studies also support that OST reduces risk of overdose death for those retained in treatment compared to those waiting for treatment or who have left treatment.
There is insufficient review evidence to support or discount the effectiveness of prison-based OST and overdose prevention. Findings from one post-RCT follow-up study suggest that retention in prison based OST after release was associated with reduced mortality.
Supervised drug consumption facilities
Effects on HIV and HCV incidence/prevalence
A supplementary review (Hedrich, 2004) identified two linked studies that examined the effect of DCR on operation BBV incidence/prevalence in Sydney (MSIC Evaluation Committee, 2003). No evidence of an increase or decrease in the incidence of notifications for HIV, HCV or HBV infections in the DCR locality compared to control localities were attributable to the operation of the DCR (MSIC Evaluation Committee, 2003). It was acknowledged a priori that low population prevalence of these infections and the limited coverage of one DCR made it unlikely there would be a detectable community-level impact on BBV incidence (MSIC Evaluation Committee, 2003). Complimentary case-control and serial cross-sectional studies of HCV incidence and HCV prevalence respectively among IDUs in the DCR locality found HCV incidence was stable and that a trend towards increased HCV prevalence was consistent with national trends among IDUs (MSIC Evaluation Committee, 2003).
Hedrich (2004) concluded:
Few data are available regarding the impact of the rooms on the incidence of infectious diseases among clients. Methodologically, it is difficult to establish a causal effect of the rooms per se that can be distinguished from the effects of the gamut of health promotion and harm reduction activities aimed at preventing drug-related infectious diseases.
(Hedrich, 2004, p. 77)
Evidence statement for DCRs and HIV/HCV incidence/prevalence
There is insufficient review-level or primary evidence to support or discount the effect of DCRs on HIV or HCV prevalence/incidence.
Effects on injecting risk behaviour
Two supplementary reviews (Hedrich, 2004; Kerr et al., 2007), a synthesis of Vancouver evaluation findings (Wood et al., 2006) and a meta-analysis (Milloy and Wood, 2009) examined evidence of the effect of DCR use on self-reported (and in some instances staffreported)
injecting risk behaviour.
Hedrich (2004) identified 13 studies of DCRs and injecting risk behaviour (Benninghoff and Dubois-Arber, 2002; Benninghoff et al., 2003; Jacob et al., 1999; Linssen et al., 2000; Meijer et al., 2001; Minder Nejedly and Bürki, 1996; MSIC Evaluation Committee, 2003; Poschadel et al., 2003; Reyes Fuentes, 2003; Ronco et al., 1996; van der Poel et al., 2003; Zurhold et al., 2001). These comprised mostly serial and single cross-sectional studies with small sample sizes. However, they consistently showed a positive impact of DCR use on injecting-related risk behaviour, including: improved knowledge and/or practice of injecting hygiene and safer use (Benninghoff and Dubois-Arber, 2002; Benninghoff et al., 2003; Jacob et al., 1999; Linssen et al., 2000; Meijer et al., 2001; MSIC Evaluation Committee, 2003; Poschadel et al., 2003; van der Poel et al., 2003; Zurhold et al., 2001); increased use of sterile injecting equipment for all injections (Minder Nejedly and Bürki, 1996; MSIC Evaluation Committee, 2003; Reyes Fuentes, 2003; Ronco et al., 1996); decreases in needle syringe and other equipment sharing (Benninghoff and Dubois-Arber, 2002; Benninghoff et al., 2003; Dubois- Arber et al., 1999; MSIC Evaluation Committee, 2003).
Hedrich concluded:
Clients of consumption rooms report improved knowledge of safer use and injection techniques as well as reductions in risk behaviour. Positive behavioural changes are confirmed by staff, although this process is sometimes slow. Despite methodological limitations, it is likely that safer use education given at consumption rooms has contributed to this. Effects increase with length and frequency of service use and behaviour changes are sustained outside the facilities.
(Hedrich, 2004, p. 77)
Kerr et al. (2007) referred to seven of the same studies as Hedrich (2004) (Benninghoff and Dubois-Arber, 2002; Benninghoff et al., 2003; Jacob et al., 1999; Meijer et al., 2001; Minder Nejedly and Bürki, 1996; Ronco et al., 1996; van der Poel et al., 2003) and Kerr et al. (2007) and Wood et al. (2006) both referred to three studies of DCR use and injecting risk behaviour from the prospective IDU cohort studies of the Vancouver evaluation (Kerr et al., 2005; Stoltz et al., 2007; Wood et al., 2005). The prevalence of syringe sharing decreased in
the cohort after the facility opened and only among DCR users (Kerr et al., 2005). Regular DCR use was associated with reduced syringe lending by HIV-infected IDUs and reduced syringe borrowing by HIV-negative IDUs (Wood et al., 2005). DCR use was independently
associated with decreased reuse of syringes, increased use of sterile water and increased use of alcohol swabbing of injection sites (Stoltz et al., 2007).
Milloy and Wood (2009) combined the effects of DCR use on syringe sharing from Canadian (Kerr et al., 2005; Wood et al., 2005) and Spanish (Bravo et al., 2009) cohort studies. Their pooled estimate of 0.31 (95 % confidence interval 0.17-0.55) represented a 69 % reduction in the likelihood of syringe sharing among DCR users.
Evidence statement for DCRs and injecting risk behaviour
Based on consistent evidence from multiple studies identified in two supplementary reviews and a meta-analysis we conclude there is tentative review-level evidence that DCR use is associated with reduced injecting risk behaviour and improvements in injecting practices and hygiene, especially for injections that occur on DCR premises, and among those who are regular DCR users.
Effects on overdose mortality
Two supplementary reviews (Hedrich, 2004; Kerr et al., 2007) identified an ecological study of DCRs and overdose mortality. A time series study of drug-related deaths in four German cities found a significant association between the operation of DCRs (often in multiple sites) and the reduction of drug-related deaths (Poschadel et al., 2003). Hedrich (2004) also described another time series study of DCR operation and overdose deaths and ambulance call-outs to suspected opioid overdoses in Sydney, which was inconclusive due to
confounding changes in the drug market after the opening of the DCR that led to a significant reduction in heroin use (MSIC Evaluation Committee, 2003).
Potential deaths prevented by DCR operation have also been estimated. Hedrich (2004) reported on a multiplier estimation study from Sydney that suggested that clinical intervention staff prevented at least four deaths per year (MSIC Evaluation Committee, 2003). Hedrich
(2004) also applied a mortality rate of 2 % to data on annual supervised drug consumption episodes in Germany (Poschadel et al., 2003), assuming that one ‘person year of active use’ equals 1 000 consumptions, and estimated that at least 10 deaths per year were prevented by the operation of DCRS in Germany.
Hedrich (2004) concluded:
There is some evidence ... that consumption rooms can contribute to a reduction in drug-related deaths at community level. The robustness of these analyses remains to be verified by further research data based on longitudinal analyses in different contexts that reproduce these results across time or geographic location … There is no evidence at all that consumption rooms contribute to increased morbidity or mortality risks among drug users. Millions of drug consumptions have been supervised and thousands of emergencies been treated — with no deaths from overdose.
(Hedrich, 2004, p. 77)
Evidence statement for DCRs and overdose deaths
There is insufficient review-level evidence to support or discount the effect of DCRs on reduction of overdose deaths at the community level. One time-series study found DCR operation was associated with reduced drug-related deaths at a city level. Process data show no overdose deaths have occurred on DCR premises and clinical and epidemiological data suggest it is likely that a proportion of overdoses treated in DCR settings would have been fatal if they had occurred elsewhere.
Peer naloxone distribution
We identified one supplementary review of PND to reduce heroin deaths (Baca and Grant, 2005), which reported limited process outcomes of two early PND programmes (Bigg, 2002; Dettmer et al., 2001).
The process evaluation literature on PND has grown considerably since that review and we identified nine subsequently published primary studies (Galea et al., 2006; Green et al., 2008; Piper et al., 2008; Seal et al., 2005; Sherman et al., 2009; Strang et al., 2008; Tobin
et al., 2009; Wagner et al., 2009).
Taken together, the evidence from four prospective studies (Seal et al., 2005; Strang et al., 2008; Tobin et al., 2009; Wagner et al., 2009) and three cross-sectional studies (Green et al., 2008; Piper et al., 2008; Sherman et al., 2009) suggests that overdose prevention training with PND increases participants’ knowledge, confidence and skills to respond effectively in case of overdose. Evidence from five prospective studies (Galea et al., 2006; Seal et al., 2005; Strang et al., 2008; Tobin et al., 2009; Wagner et al., in press) and three crosssectional studies (Dettmer et al., 2001; Piper et al., 2008) suggests PND trainees subsequently intervene at overdose using naloxone with very high reported rates of survival in cases where the outcome of intervention is known.
Effects on overdose mortality
We identified one ecological study that examined the impact of PND on overdose mortality at the community level. In Chicago, a large-scale PND programme has been operating since 2001 with more than 3 500 vials of naloxone prescribed and 319 naloxone reversals
reported by programme participants. Coronial data showed that the upward trend in heroin overdose deaths annually in Chicago prior to the PND programme, which increased fourfold between 1996 and 2000, reversed in 2001, with a 20 % decrease in 2001 and a 10 %
decreases in 2002 and 2003 (Maxwell et al., 2006).
Evidence statement for PND and overdose
There is insufficient review-level evidence to draw conclusions about the effect of PND on overdose deaths. Recently published primary studies consistently point to the feasibility and uptake of PND programmes. One ecological study suggests the operation of a large PND
programme may have played a role in reducing overdose deaths at the city level.
Discussion
Drawing substantively upon our previous work in this area (Palmateer et al., 2008; Palmateer et al., 2010), we have used a review of reviews methodology to evaluate the evidence relating to the effectiveness of selected harm reduction interventions on key indicators of injecting-related morbidity and mortality: NSPs, OST, DCRs on HIV and HCV incidence/prevalence and injecting risk behaviour; and OST, DCRs, and PND on overdose-related deaths.
We find that there is sufficient review-level evidence that OST reduces HIV transmission, while the review evidence in support of NSPs reducing HIV transmission is more tentative, and for DCRs currently insufficient. We find there is tentative review-level evidence that OST has limited effectiveness in reducing HCV transmission, and insufficient evidence to support or discount that NSPs or DCRs reduce HCV transmission. We find there is sufficient review-level evidence that NSPs, OST and DCRs reduce self-reported injecting risk behaviour and tentative review-level evidence to suggest that pharmacy access, in addition to primary NSP, is effective in reducing injecting risk behaviour. There is sufficient review-level evidence that OST is effective in reducing opioid overdose related mortality but insufficient review-level evidence to support or discount the effectiveness of DCRs and PND in reducing overdose deaths at the community level.
Our findings highlight a lack of high-quality reviews for some harm reduction interventions and/or outcomes we considered. In some cases this reflects a lack of primary studies (e.g. DCRs and PND). It also appears that previous reviews of NSPs may have overstated the evidence of effectiveness in BBV prevention from the available studies. In general, we found that reviews gave more consideration to issues of bias and limitations in studies with negative findings than in studies with positive (protective) findings, and thus may have ascribed less importance to negative studies when synthesising the evidence
(Palmateer et al., in press).
As highlighted earlier, an assessment of insufficient or tentative review-level evidence does not equate to evidence for lack of intervention effectiveness. Such assessments are inevitably related to the methodological limitations of primary studies as well as the reviews (Palmateer et al., 2010). For example, one of the criticisms of studies investigating NSPs’ effectiveness in preventing BBVs is that they do not accurately measure the coverage or intensity of the intervention delivered (that is, the amount of injecting equipment distributed) (Lurie, 1997).
Further consideration of the limitations of the primary studies helps to explain our finding of a discrepancy between the results of individual-level (i.e. cohort and case-control) and ecological studies of NSP effectiveness (Palmateer et al., 2010). First, individual-level, nonrandomised studies are highly susceptible to bias. In cohort studies, for example, two groups, such as NSP attenders and non-attenders, are usually compared to the outcome. This measurement of the exposure to the intervention has generally been limited because: (i) these groups are ‘self-selecting’ and thus may be inherently different with respect to characteristics, including injecting risk, that can influence the outcome (Lurie, 1997), and (ii) the distinction between exposed and unexposed groups may also be inadequate (for example, unexposed individuals may have access to clean needles/syringes from other sources or exposed individuals may still be engaging in injecting risk despite high uptake of NSP), potentially diluting the effect size (Gibson et al., 2001).
Ecological studies, by contrast, are more likely to report a positive association: because one cannot isolate the effects of a single intervention in an ecological study, such studies may in fact be measuring the impact of several interventions. This is illustrated in the Amsterdam Cohort Study (ACS), which found that MMT or NSP use alone were not associated significantly with HIV or HCV seroconversion, but that full participation in both programmes was associated with a lower incidence of HCV and HIV infection, suggesting that only the combination of these interventions might contribute to the reduction of the transmission of these infections (van den Berg et al., 2007).
All of the evidence for NSP, DCR and PND effectiveness is based on observational study designs, that is, exposure has not been randomised. Observational studies, as discussed above, are generally at risk of confounding and selection bias. However, it is logistically
and ethically difficult to conduct a randomised trial for interventions such as NSPs and DCRs, which have face validity and have already been widely introduced (Hall and Kimber, 2005; Lurie, 1997). A feasible alternative study design is a community-randomised trial (e.g. comparing a basic package of harm reduction services with an enhanced package) where participants are randomised on a group basis, rather than an individual basis, thereby avoiding some of the biases associated with observational designs (Tilson et al., 2007).
Another methodological issue is that the primary studies might not have been adequately powered to detect an impact. Few of the reviews addressed this issue in their reporting of the studies and, therefore, it was usually unclear whether equivocal findings were due to a lack of power or truly represented no association (Palmateer et al., 2010).
The reliance on self-reported behaviour is a problem for epidemiological studies examining the effectiveness of harm reduction interventions. Self-reported behaviour by drug users can be reliable (Darke, 1988; Goldstein et al., 1995); however, it is unclear whether this applies to all behaviours. Limitations, for example, in the reliability selfreported injecting risk behaviour may explain our finding of greater strength of evidence for behavioural measures than for biological measures. Differential reporting of risk behaviour between exposed and unexposed groups could bias measures of the effectiveness of an intervention, for example if IDUs exposed to NSPs are more sensitised to the risks of sharing and more reluctant to report this behaviour than unexposed individuals (Palmateer et al., 2010). Second, some modelling studies (Vickerman et al., 2006) have suggested that the association between injecting risk behaviour and HIV/ HCV transmission does not follow a dose-response relationship; rather, a reduction in injecting risk has to surpass a threshold level before changes in HIV/HCV transmission are observed. Consequently, a sub-threshold change in injecting risk behaviour may have no impact on HIV/HCV incidence, thereby limiting the usefulness of injecting risk behaviour as a proxy measure for the effectiveness of an intervention (Palmateer et al., 2010).
We acknowledge that we may have missed potentially relevant reviews by limiting our search to English language reviews, although we attempted to expand the search, and reduce publication bias, by examining the grey literature. In particular in the reviews of DCRs, prison NSPs and prison OST there is good coverage of non-English language studies. We also aimed to address potential gaps in the review evidence by undertaking searches of recently published primary literature.
Another limitation of the review of reviews methodology is the reliance on the reviewers’ identification of the relevant studies and their accounts of the designs and findings of the primary studies. In considering the primary evidence, we used the study design as a proxy
for study quality; however, other factors — for example sample size and recruitment strategy — affect the integrity of a study’s results. The likelihood of having missed primary studies is a possibility for outcomes that core reviews did not specifically set out to examine: we
attempted to compensate for this by including the studies identified by supplementary reviews (Palmateer et al., 2010).
We have also focused our evaluation of harm reduction programmes on a subset of interventions and outcomes. This is not to suggest that other interventions (e.g. education information and counselling) or outcomes (e.g. health and social functioning) that we have
not examined are not important components of these programmes. Additionally, we have focused on the ‘direct’ evidence of effectiveness of the selected interventions (that is, changes in biological or behavioural outcomes).
Implications for harm reduction practice and evaluation
In most European countries, harm reduction interventions developed in response to community-level identified needs, and were often introduced in the absence of methodologically rigorous evaluation. We have found the quality of evidence on intervention impacts to be lacking in some cases, but this is not uncommon for behavioural interventions in public health more generally and harm reduction interventions in HIV prevention are the subject of much evaluation research. Our assessment of the quality of evidence does not suggest that policymakers should disinvest from harm reduction programmes. Rather, the provision and increase in coverage of interventions needs to be used as an opportunity to conduct better research into the effectiveness of these interventions.
Conclusions and recommendations
European countries face a challenge in reducing/maintaining low prevalence of BBVs among IDUs and reducing drug overdose mortality. Good quality research is fundamental to formulating policy on the development, scale-up and continued investment in public health
interventions targeting IDUs. We recommend a step change in evaluations of harm reduction interventions so that future evaluations: (i) include both biological and behavioural outcomes and are powered to detect changes in the outcome of interest; (ii) consider complete
packages of harm reduction interventions rather than single interventions; (iii) consider randomised, especially community-level, designs where possible, and report evaluation findings to CONSORT and TREND guidelines (Des Jarlais et al., 2004; Moher et al., 2001);
(iv) and compare additional interventions or increased coverage/intensity of interventions with current availability (Palmateer et al., 2010).
References
Abbott, P. J., Weller, S. B., Delaney, H. D. and Moore, B. A. (1998), ‘Community reinforcement approach in the treatment of opiate addicts’, American Journal of Drug and Alcohol Abuse 24 (1), pp. 17–30.
Amato, L., Davoli, M., Perucci, C. A., et al. (2005), ‘An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research’, Journal of Substance Abuse Treatment 28, pp. 321–9.
Amundsen, E. J., Eskild, A., Stigum, H., Smith, E. and Aalen, O. O. (2003), ‘Legal access to needles and syringes/needle exchange programmes versus HIV counselling and testing to prevent transmission of HIV among intravenous drug users: a comparative study of Denmark, Norway and Sweden’, European Journal of Public Health 13, pp. 252–8.
Avants, S. K., Margolin, A., Kosten, T. R., Rounsaville, B. J. and Schottenfeld, R. S. (1998), ‘When is less treatment better? The role of social anxiety in matching methadone patients to psychosocial treatments’, Journal of Consulting and Clinical Psychology 66 (6), pp. 924–31.
Baca, C. T. and Grant, K. J. (2005), ‘Take-home naloxone to reduce heroin death’, Addiction 100 (12), pp. 1823–31.
Baker, A., Kochan, N., Dixon, J., Wodak, A. and Heather, N. (1995), ‘HIV risk-taking behaviour among injecting drug users currently, previously and never enrolled in methadone treatment’, Addiction 90 (4), pp. 545–54.
Ball, J. C., Lange, W. R., Myers, R. P. and Friedman, S. (1998), ‘Reducing the risk of AIDS through methadone maintenance treatment’, Journal of Health and Social Behavior 29, pp. 214–26.
Bargagli, A. M., Hickman, M., Davoli, M., et al. (2006), ‘Drug-related mortality and its impact on adult mortality in eight European countries’, European Journal of Public Health 16 (2), pp. 198–202.
Batki, S. L., Sorensen, J. L., Gibson, D. R. and Maude-Griffin, P. (1989), ‘HIV-infected i.v. drug users in methadone treatment: outcome and psychological correlates: a preliminary report’, NIDA Research Monograph 95, pp. 405–6.
Bayanzadeh, S. A., et al. (2004), ‘A study of the effectiveness of psychopharmacological intervention in reducing harm/high risk behaviours among substance user prisoners’.
Benninghoff, F. and Dubois-Arber, F. (2002), ‘Résultats de l’étude de la clientèle du Cactus BIEL/BIENNE 2001’, Institut universitaire de médecine sociale et préventive, Lausanne.
Benninghoff, F., Solai, S., Huissoud, T. and Dubois-Arber, F. (2003), ‘Evaluation de Quai 9 “Espace d’acceuil et d’injection” à Genéve: période 12/2001–12/2000’, Institut universitaire de médecine sociale et préventive, Lausanne.
Best, D., Man, L. H., Zador, D., et al. (2000), ‘Overdosing on opiates: part II — prevention’, Drug and Alcohol Findings (5), pp. 4–18.
Bewley-Taylor, D. (2002), ‘Challenging the UN drug control conventions: problems and possibilities’, International Journal of Drug Policy 14 (2), pp. 171–9.
Bigg, D. (2002), ‘Data on take home naloxone are unclear but not condemnatory’, BMJ 324 (7338), p. 678.
Bluthenthal, R. N., Kral, A. H., Erringer, E. A. and Edlin, B. R. (1998), ‘Use of an illegal syringe exchange and injection-related risk behaviors among street-recruited injection drug users in Oakland, California, 1992 to 1995’, Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 18 (5), pp. 505–11.
Bluthenthal, R. N., Kral, A. H., Gee, L., Erringer, E. A. and Edlin, B. R. (2000), ‘The effect of syringe exchange use on high-risk injection drug users: a cohort study’, AIDS 14 (5), pp. 605–11.
Boguña, J. (1997), ‘Methadone maintenance programmes’, in O’Brien, O. (ed.), Report of the 3rd European Conference on Drug and HIV/AIDS Services in Prison, Cranstoun Drug Services, London, pp. 68–70.
Bravo, M. J., Royuela, L., Brugal, M. T., Barrio, G. and Domingo-Salvany, A. (2009), ‘Use of supervised injection facilities and injection risk behaviours among young drug injectors’, Addiction 104 (4), pp. 614–19.
Broadhead, R. S., van Hulst, Y. and Heckathorn, D. D. (1999), ‘The impact of a needle exchange’s closure’, Public Health Reports 114, pp. 439–47.
Brooner, R., Kidorf, M., King, V., et al. (1998), ‘Drug abuse treatment success among needle exchange participants’, Public Health Reports 113 Supplement 1, pp. 129–39.
Brugal, M. T., Domingo-Salvany, A., Puig, R., et al. (2005), ‘Evaluating the impact of methadone maintenance programmes on mortality due to overdose and AIDS in a cohort of heroin users in Spain’, Addiction 100 (7), pp. 981–9.
Bruneau, J., Lamothe, F., Franco, E., et al. (1997), ‘High rates of HIV infection among injection drug users participating in needle exchange programs in Montreal: results of a cohort study’, American Journal of Epidemiology 146 (12), pp. 994–1002.
Camacho, L. M., Bartholomew, N. G., Joe, G. W., Cloud, M. A. and Simpson, D. D. (1996), ‘Gender, cocaine and during-treatment HIV risk reduction among injection opioid users in methadone maintenance’, Drug and Alcohol Dependence 41 (1), pp. 1–7.
Caplehorn, J. and Ross, M. (1995), ‘Methadone maintenance and the likelihood of risky needle-sharing’, International Journal of the Addictions 30, pp. 685–98.
Caplehorn, J., Dalton, M. and Petrenas, A. (1994), ‘Retention in methadone maintenance and heroin addicts’ risk of death’, Addiction 89, pp. 203–07.
Caplehorn, J., Dalton, M. S., Haldar, F., Petrenas, A. M. and Nisbet, J. G. (1996), ‘Methadone maintenance and addicts’ risk of fatal heroin overdose’, Substance Use and Misuse 31 (2), pp. 177–96.
Caslyn, D. A. (1992), ‘Ineffectiveness of AIDS education and HIV antibody testing in reducing high risk behaviours among injecting drug users’, American Journal of Public Health 82, pp. 573–5.
Caslyn, D. A., Saxon, A., Freeman, G. and Whittaker, S. (1991), ‘Needle practices among intravenous drug users in an area where needle purchase is still legal’, AIDS 5, pp. 187–93.
Chamot, E., de Saussure, P., Hirschel, B., Deglon, J. J. and Perrin, L. H. (1992), ‘Incidence of hepatitis C, hepatitis B and HIV infections among drug users in a methadone-maintenance programme’, AIDS 6 (4), pp. 430–1.
Chatham, L. R., Hiller, M. L., Rowan-Szal, G. A., Joe, G. W. and Simpson, D. D. (1999), ‘Gender differences at admission and follow-up in a sample of methadone maintenance clients’, Substance Use and Misuse 34 (8), pp. 1137–65.
Clausen, T., Anchersen, K. and Waal, H. (2008), ‘Mortality prior to, during and after opioid maintenance treatment (OMT): a national prospective cross-registry study’, Drug and Alcohol Dependence 94, pp. 151–7.
Cook, C., Bridge, J. and Stimson, G. V. (2010), ‘The diffusion of harm reduction in Europe and beyond’, in European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), Harm reduction: evidence, impacts and challenges, Rhodes, T. and Hedrich, D. (eds), Scientific Monograph Series No. 10, Publications Office of the European Union, Luxembourg.
Coutinho, R. (2005), ‘Needle exchange: the Amsterdam experience’, in Institute of Medicine workshop on the prevention of HIV among injecting drug users in high-risk countries, Institute of Medicine Committee on the Prevention of HIV Infection Among Injecting Drug Users in High-Risk Countries, Geneva.
Cox, G. M., Lawless, M. C., Cassin, S. P. and Geoghegan, T. W. (2000), ‘Syringe exchanges: a public health response to problem drug use’, Irish Medical Journal 93, pp. 143–6.
Craine, N., Hickman, M., Parry, J. V., et al. (2009), ‘Incidence of hepatitis C in drug injectors: the role of homelessness, opiate substitution treatment, equipment sharing, and community size’, Epidemiology and Infection 137 (9), pp. 1255–65.
Crofts, N., Nigro, L., Oman, K., Stevenson, E. and Sherman, J. (1997), ‘Methadone maintenance and hepatitis C virus infection among injecting drug users’, Addiction 92 (8), pp. 999–1005.
Cushman, P. J. (1977), ‘Ten years of methadone maintenance treatment: some clinical observations’, American Journal of Drug and Alcohol Abuse 4, pp. 543–53.
Darke, S. (1988), ‘Self-report among injecting drug users: a review’, Drug and Alcohol Dependence 51 (3), pp. 253–63.
Darke, S. and Hall, W. (1997), ‘The distribution of naloxone to heroin users’, Addiction 92 (9), pp. 1195–9.
Darke, S. and Hall, W. (2003), ‘Heroin overdose: research and evidence-based intervention’, Journal of Urban Health 80 (2), pp. 189–200.
Darke, S. and Ross, J. (1999), ‘Heroin-related deaths in south western Sydney: 1992–1996’, Drug and Alcohol Review 18, pp. 39–45.
Darke, S., Ross, J., Zador, D. and Sunjic, S. (2000), ‘Heroin-related deaths in New South Wales, Australia, 1992–1996’, Drug and Alcohol Dependence 60, pp. 141–50.
Davoli, M., Perucci, C. A., Forastiere, F., et al. (1993), ‘Risk factors for overdose mortality: a case control study within a cohort of intravenous drug users’, International Journal of Epidemiology 22 (2), pp. 272–7.
Davoli, M., Bargagli, A. M., Perucci, C. A., et al. (2007), ‘Risk of fatal overdose during and after specialist drug treatment: the VEdeTTE study, a national multi-site prospective cohort study’, Addiction 102 (12), pp. 1954–9.
De Jong, W., Tsagarelli, T. and Schouten, E. (1999), ‘Rapid assessment of injection drug use and HIV in the Republic of Georgia’, Journal of Drug Issues 29 (4), pp. 843–60.
Degenhardt, L., Hall, W., Lynskey, M. and Warner-Smith, M. (2004), ‘Illicit drug use’, in Ezzati, M., Lopez, A., Rodgers, A. and Murray, C. (eds), Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors, World Health Organization, Geneva.
Degenhardt, L., Hall, W. and Warner-Smith, M. (2006), ‘Using cohort studies to estimate mortality among injecting drug users that is not attributable to AIDS’, Sexually Transmitted Infections 82, pp. 56–63.
Degenhardt, L., Randall, D., Hall, W., et al. (2009), ‘Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved’, Drug and Alcohol Dependence 105 (1–2), pp. 9–15.
Des Jarlais, D. C., Friedman, S. R., Sotheran, J. L., et al. (1994), ‘Continuity and change within an HIV epidemic: injecting drug users in New York City, 1984 through 1992’, JAMA 271 (2), pp. 121–7.
Des Jarlais, D. C., Hagan, H., Friedman, S. R., et al. (1995), ‘Maintaining low HIV seroprevalence in populations of injecting drug users’, JAMA 274 (15), pp. 1226–31.
Des Jarlais, D. C., Marmor, M., Paone, D., et al. (1996), ‘HIV incidence among injecting drug users in New York City syringe-exchange programmes’, Lancet 348 (9033), pp. 987–91.
Des Jarlais, C., Perlis, T., Friedman, S. R., et al. (2000), ‘Behavioral risk reduction in a declining HIV epidemic: injection drug users in New York City, 1990–1997’, American Journal of Public Health 90 (7), pp. 1112–16.
Des Jarlais, D., Lyles, C., Crepaz, N. and TREND Group (2004), ‘Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement’, American Journal of Public Health 94 (3), pp. 361–6.
Des Jarlais, D. C., Perlis, T., Arasteh, K., et al. (2005a), ‘HIV incidence among injection drug users in New York City, 1990 to 2002: use of serologic test algorithm to assess expansion of HIV prevention services’, American Journal of Public Health 95 (8), pp. 1439–44.
Des Jarlais, D. C., Perlis, T., Arasteh, K., et al. (2005b), ‘Reductions in hepatitis C virus and HIV infections among injecting drug users in New York City, 1990–2001’, AIDS 19 (Supplement 3), pp. S20–S25.
Dettmer, K., Saunders, B. and Strang, J. (2001), ‘Take home naloxone and the prevention of deaths from opiate overdose: two pilot schemes’, British Medical Journal 322 (7291), pp. 895–6.
Dolan, K., Hall, W. and Wodak, A. (1996), ‘Methadone maintenance reduces injecting in prison’, British Medical Journal 312, p. 1162.
Dolan, K., Wodak, A. and Hall, W. (1998), ‘Methadone maintenance treatment reduces heroin injection in NSW prisons’, Drug and Alcohol Review 17 (2), pp. 153–8.
Dolan, K., Rutter, S. and Wodak, A. D. (2003a), ‘Prison-based syringe exchange programmes: a review of international research and development’, Addiction 98 (2), pp. 153–8.
Dolan, K. A., Shearer, J., MacDonald, M., et al. (2003b), ‘A randomised controlled trial of methadone maintenance treatment versus wait list control in an Australian prison system’, Drug and Alcohol Dependence 72 (1), pp. 59–65.
Dolan, K. A., Shearer, J., White, B., et al. (2005), ‘Four-year follow-up of imprisoned male heroin users and methadone treatment: mortality, re-incarceration and hepatitis C infection’, Addiction 100 (6), pp. 820–8.
Donoghoe, M. C., Stimson, G. V., Dolan, K. and Alldritt, L. (1989), ‘Changes in HIV risk behaviour in clients of syringe-exchange schemes in England and Scotland’, AIDS 3 (5), pp. 267–72.
Donoghoe, M. C., Dolan, K. and Stimson, G. V. (1992), ‘Life-style factors and social circumstances of syringe sharing in injecting drug users’, British Journal of Addiction 87, pp. 993–1003.
Dubois-Arber, F., Jeannin, A. and Spencer, B. (1999), ‘Evaluation of the AIDS prevention strategy in Switzerland (6th synthesis report 1996–1998)’, Institut universitaire de medicine sociale e preventitive, Lausanne.
Ellis, S., Barnett-Page, E., Morgan, A., et al. (2003), HIV prevention: a review of reviews assessing the effectiveness of interventions to reduce the risk of sexual transmission, Health Development Agency, London.
EMCDDA (2008), The state of the drugs problem in Europe: annual report 2008, European Monitoring Centre for Drugs and Drug Addiction, Lisbon.
EMCDDA (2009), The state of the drugs problem in Europe: annual report 2009, European Monitoring Centre for Drugs and Drug Addiction, Lisbon.
Emmanuelli, J. and Desenclos, J. C. (2005), ‘Harm reduction interventions, behaviours and associated health outcomes in France, 1996–2003’, Addiction 100 (11), pp. 1690–700.
European Commission (2007), Report from the Commission to the European Parliament and the Council on the implementation of the Council recommendation of 18 June 2003 on the prevention and reduction of health-related harm associated with drug dependence, COM (2007) 199 final, Commission of the European Communities, Brussels.
Faggiano, F., Vigna-Taglianti, F., Versino, E. and Lemma, P. (2003), ‘Methadone maintenance at different dosages for opioid dependence’, Cochrane Database of Systematic Reviews 3, p. CD002208.
Frischer, M. and Elliott, L. (1993), ‘Discriminating needle exchange attenders from non-attenders’, Addiction 88 (5), pp. 681–7.
Fugelstad, A., Rajs, J., Böttiger, M. and Gerhardsson de Verdier, M. (1995), ‘Mortality among HIV-infected intravenous drug addicts in Stockholm in relation to methadone treatment’, Addiction 90 (5), pp. 711–16.
Galea, S., Worthington, N., Piper, T. M., et al. (2006), ‘Provision of naloxone to injection drug users as an overdose prevention strategy: early evidence from a pilot study in New York City’, Addictive Behaviors 31 (5), pp. 907–12.
Gearing, F. R. and Schweitzer, M. D. (1974), ‘An epidemiologic evaluation of long term methadone maintenance treatment for heroin addiction’, American Journal of Epidemiology 100, pp. 101–12.
Gibson, D. R., Flynn, N. M. and Perales, D. (2001), ‘Effectiveness of syringe exchange programs in reducing HIV risk behavior and HIV seroconversion among injecting drug users’, AIDS 15 (11), pp. 1329–41.
Gibson, D. R., Brand, R., Anderson, K., et al. (2002), ‘Two- to sixfold decreased odds of HIV risk behavior associated with use of syringe exchange’, Journal of Acquired Immune Deficiency Syndromes 31 (2), pp. 237–42.
Glaziou, P. and Heneghan, C. (2009), ‘A spotter’s guide to study designs’, Evidence Based Medicine 14, pp. 37–8.
Gleghorn, A. A., Jones, T. S., Doherty, M. C., Celentano, D. D. and Vlahov, D. (1995), ‘Acquisition and use of needles and syringes by injecting drug users in Baltimore, Maryland’, Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 10 (1), pp. 97–103.
Gleghorn, A. A., Wright-De Aguero, L. and Flynn, C. (1998), ‘Feasibility of one-time use of sterile syringes: a study of active injection drug users in seven United States metropolitan areas’, Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 18 (Supplement 1), pp. S30–S36.
Goldberg, D., Cameron, S. and McMenamin, J. (1998), ‘Hepatitis C virus antibody prevalence among injecting drug users in Glasgow has fallen but remains high’, Communicable Disease and Public Health 1 (2), pp. 95–7.
Goldberg, D., Burns, S., Taylor, A., et al. (2001), ‘Trends in HCV prevalence among injecting drug users in Glasgow and Edinburgh during the era of needle/syringe exchange’, Scandinavian Journal of Infectious Diseases 33 (6), pp. 457–61.
Goldstein, M. F., Friedman, S. R., Neaigus, A., et al. (1995), ‘Self-reports of HIV risk behavior by injecting drug users: are they reliable?’ Addiction 90 (8), pp. 1097–104.
Gossop, M., Marsden, J., Stewart, D. and Rolfe, A. (2000), ‘Patterns of improvement after methadone treatment: 1 year follow-up results from the National Treatment Outcome Research Study’, Drug and Alcohol Dependence 60 (3), pp. 275–86.
Gowing, L., Farrell, M., Bornemann, R., Sullivan, L. and Ali, R. (2008), ‘Substitution treatment of injecting opioid users for prevention of HIV infection’, Cochrane Database of Systematic Reviews (2), DOI: 10.1002/14651858. CD004145.pub3.
Green, T. C., Heimer, R. and Grau, L. E. (2008), ‘Distinguishing signs of opioid overdose and indication for naloxone: an evaluation of six overdose training and naloxone distribution programs in the United States’, Addiction 103 (6), pp. 979–89.
Greenfield, L., Bigelow, G. E. and Brooner, R. (1995), ‘Validity of intravenous drug abusers’ self-reported changes in HIV high-risk drug use behaviors’, Drug and Alcohol Dependence 39 (2), pp. 91–98.
Grella, C. E., Anglin, M. D., Rawson, R., Crowley, R. and Hasson, A. (1996), ‘What happens when a demonstration project ends: consequences for a clinic and its clients’, Journal of Substance Abuse Treatment 13 (3), pp. 249–56.
Gronbladh, L., Ohlund, L. S. and Gunne, L. M. (1990), ‘Mortality in heroin addiction: impact of methadone treatment’, Acta Psychiatrica Scandinavica 82, pp. 22–37.
Groseclose, S. L., Weinstein, B., Jones, T. S., et al. (1995), ‘Impact of increased legal access to needles and syringes on practices of injecting-drug users and police officers: Connecticut, 1992–1993’, Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 10 (1), pp. 82–9.
Gunne, L. M. and Gronbladh, L. (1981), ‘The Swedish methadone maintenance program: a controlled study’, Drug and Alcohol Dependence 7, pp. 249–56.
Guydish, J., Clark, G., Garcia, D. and Bucardo, J. (1995), ‘Evaluation of needle exchange using street-based survey methods’, Journal of Drug Issues 25, pp. 33–41.
Guydish, J., Bucardo, J., Clark, G. and Bernheim, S. (1998), ‘Evaluating needle exchange: a description of client characteristics, health status, program utilization, and HIV risk behavior’, Substance Use and Misuse 33 (5), pp. 1173–96.
Hagan, H. and Thiede, H. (2000), ‘Changes in injection risk behavior associated with participation in the Seattle needle-exchange program’, Journal of Urban Health 77 (3), pp. 369–82.
Hagan, H., Des Jarlais, D. C., Purchase, D., et al. (1993), ‘An interview study of participants in the Tacoma, Washington, syringe exchange’, Addiction 88 (12), pp. 1691–7.
Hagan, H., Des Jarlais, D. C. and Friedman, S. (1994), Risk for human immunodeficiency virus and hepatitis B virus in users of the Tacoma syringe exchange program, National Academy Press, Washington, DC.
Hagan, H., Des Jarlais, D. C., Friedman, S. R., Purchase, D. and Alter, M. J. (1995), ‘Reduced risk of hepatitis B and hepatitis C among injection drug users in the Tacoma syringe exchange program’, American Journal of Public Health 85 (11), pp. 1531–7.
Hagan, H., McGough, J. P., Thiede, H., et al. (1999), ‘Syringe exchange and risk of infection with hepatitis B and C viruses’, American Journal of Epidemiology 149 (3), pp. 203–13.
Hagan, H., Thiede, H. and Des Jarlais, D. C. (2004), ‘Hepatitis C virus infection among injection drug users: survival analysis of time to seroconversion’, Epidemiology 15 (5), pp. 543–9.
Hall, W. and Kimber, J. (2005), ‘Being realistic about benefits of supervised injecting facilities’, Lancet 366 (9482), pp. 271–2.
Hallinan, R., Byrne, A., Amin, J. and Dore, G. J. (2004), ‘Hepatitis C virus incidence among injecting drug users on opioid replacement therapy’, Australian and New Zealand Journal of Public Health 28 (6), pp. 576–8.
Hammett, T. M., Kling, R., Johnston, P., et al. (2006), ‘Patterns of HIV prevalence and HIV risk behaviors among injection drug users prior to and 24 months following implementation of cross-border HIV prevention interventions in northern Vietnam and southern China’, AIDS Education and Prevention 18 (2), pp. 97–115.
Hart, G. J., Carvell, A. L., Woodward, N., et al. (1989), ‘Evaluation of needle exchange in central London: behaviour change and anti-HIV status over one year’, AIDS 3 (5), pp. 261–5.
Hartel, D. M. and Schoenbaum, E. (1998), ‘Methadone treatment protects against HIV infections: two decades of experience in the Bronx, New York City’, Public Health Reports 113 (Supplement 1), pp. S107–S115.
Hartgers, C., Buning, E. C., van Santen, G. W., Verster, A. D. and Coutinho, R. A. (1989), ‘The impact of the needle and syringe-exchange programme in Amsterdam on injecting risk behaviour’, AIDS 3 (9), pp. 571–6.
Hartgers, C., van Ameijden, E. J., van den Hoek, J. A. and Coutinho, R. A. (1992), ‘Needle sharing and participation in the Amsterdam Syringe Exchange program among HIV-seronegative injecting drug users’, Public Health Reports 107 (6), pp. 675–81.
Health Outcomes International, National Centre in HIV Epidemiology & Clinical Research and Drummond, M. (2002), Return on investment in needle & syringe programs, Commonwealth Department of Health and Aging, Canberra.
Hedrich, D. (2004), European report on drug consumption rooms, European Monitoring Centre for Drugs and Drug Addiction, Lisbon.
Hedrich, D., Kerr, T. and Dubois-Arber, F. (2010), ‘Drug consumption facilities in Europe and beyond’, in European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), Harm reduction: evidence, impacts and challenges, Rhodes, T. and Hedrich, D. (eds), Scientific Monograph Series No. 10, Publications Office of the European Union, Luxembourg.
Heimer, R., Cantani, H., Newman, R. G., Zambrano, J., Brunet, A. and Ortiz, A. (2006), ‘Methadone maintenance in prison: evaluation of a pilot program in Puerto Rico’, Drug and Alcohol Dependence 83 (2), pp. 122–9.
Heimer, R., Kaplan, E. H., Khoshnood, K., Jariwala, B. and Cadman, E. C. (1993), ‘Needle exchange decreases the prevalence of HIV-1 proviral DNA in returned syringes in New Haven, Connecticut’, American Journal of Medicine 95 (2), pp. 214–20.
Heimer, R., Khoshnood, K., Bigg, D., Guydish, J. and Junge, B. (1998), ‘Syringe use and reuse: effects of syringe exchange programs in four cities’, Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 18 (Supplement 1), pp. S37–S44.
Heimer, R. , Zambrano, J. A., Brunet, A. et al. (2005), ‘Methadone maintenance treatment in a men’s prison in Puerto Rico: a pilot program’, Journal of Correctional Healthcare 11 (3), pp. 295–305.
Heinemann, A. and Gross, U. (2001), ‘Prevention of blood-borne virus infections among drug users in an open prison by syringe vending machines’, Sucht 47 (2), pp. 57–65.
Hernandez-Aguado, I., Ramos-Rincon, J. M., Avinio, M. J., et al. (2001), ‘Measures to reduce HIV infection have not been successful to reduce the prevalence of HCV in intravenous drug users’, European Journal of Epidemiology 17 (6), pp. 539–44.
Hunter, G. M., Donoghoe, M. C., Stimson, G. V., Rhodes, T. and Chalmers, C. P. (1995), ‘Changes in the injecting risk behaviour of injecting drug users in London, 1990–1993’, AIDS 9 (5), pp. 493–501.
Huo, D., Bailey, S. L., Garfein, R. S. and Ouellet, L. J. (2005), ‘Changes in the sharing of drug injection equipment among street-recruited injection drug users in Chicago, Illinois, 1994–1996’, Substance Use and Misuse 40 (1), pp. 63–76.
Hurley, S. F., Jolley, D. J. and Kaldor, J. M. (1997), ‘Effectiveness of needle-exchange programmes for prevention of HIV infection’, Lancet 349 (9068), pp. 1797–800.
Hutchinson, S. J., McIntyre, P. G., Molyneaux, P., et al. (2002), ‘Prevalence of hepatitis C among injectors in Scotland 1989–2000: declining trends among young injectors halt in the late 1990s’, Epidemiology and Infection 128 (3), pp. 473–7.
Iguchi, M. Y. (1998), ‘Drug abuse treatment as HIV prevention: changes in social drug use patterns might also reduce risk’, Journal of Addictive Diseases 17 (4), pp. 9–18.
Ingold, F. R. and Ingold, S. (1989), ‘The effects of the liberalization of syringe sales on the behavior of intravenous drug users in France’, Bulletin on Narcotics 41, pp. 67–81.
Islam, M. M. and Conigrave, K. M. (2007), ‘Assessing the role of syringe dispensing machines and mobile van outlets in reaching hard-to-reach and high-risk groups of injecting drug users (IDUs): a review’, Harm Reduction Journal 4 (14), DOI: 10.1186/1477-7517-4-14.
Jacob, J. and Stöver, H. (1997), ‘Clean needles for Saxon prisoners’, Prison Report Spring, pp. 22–3.
Jacob, J. and Stöver, H. (2000), ‘Drug use, drug control, and drug services in German prisons: contradictions, insufficiencies and innovative approaches’, in Shewan, D. and Davies, J. (eds), Drug use and prisons: an international perspective, Overseas Publishers Association, Amsterdam, pp. 57–87.
Jacob, J., Rottman, J. and Stöver, H. (1999), Entstehung und Praxis eines Gesundheitsraumangebotes für Drogenkonsumierende. Abschlußbericht der einjährigen Evaluation des ‘drop-in Fixpunkt’, Hannover, Bibliotheks und Informationssystem der Universität Oldenburg, Oldenburg.
Jones, L., Pickering, L., Sumnall, H., McVeigh, J. and Bellis, M. A. (2008), A review of the effectiveness and costeffectiveness of needle and syringe programmes for injecting drug users, Centre for Public Health, John Moores University, Liverpool.
Käll , K., Hermansson, U., Amundsen, E. J. and Ronnback, S. (2007), ‘The effectiveness of needle exchange programmes for HIV prevention: a critical review’, Journal of Global Drug Policy and Practice 1. Available at http://www.globaldrugpolicy.org/1/3/1.php (accessed 20 October 2009).
Keene, J., Stimson, G. V., Jones, S. and Parry-Langdon, N. (1993), ‘Evaluation of syringe-exchange for HIV prevention among injecting drug users in rural and urban areas of Wales’, Addiction 88 (8), pp. 1063–70.
Kelly, M., Swann, C., Killoran, A., et al. (2002), Methodological problems in constructing the evidence base in public health, Health Development Agency, London.
Kerr, T., Tyndall, M., Li, K., Montaner, J. and Wood, E. (2005), ‘Safer injection facility use and syringe sharing in injection drug users’, Lancet 366 (9482), pp. 316–18.
Kerr, T., Kimber, J., De Beck, K. and Wood, E. (2007), ‘The role of safer injection facilities in the response to HIV/AIDS among injection drug users’, Current HIV Reports 4, pp. 158–64.
Kimber, J., Dolan, K., van Beek, I., Hedrich, D. and Zurhold, H. (2003), ‘Drug consumption facilities: an update since 2000’, Drug and Alcohol Review 22, pp. 227–33.
King, V. L., Kidorf, M. S., Stoller, K. B. and Brooner, R. K. (2000), ‘Influence of psychiatric comorbidity on HIV risk behaviors: changes during drug abuse treatment’, Journal of Addictive Diseases 19 (4), pp. 65–83.
Klee, H. and Morris, J. (1995), ‘The role of needle exchanges in modifying sharing behaviour: cross-study comparisons 1989–1993’, Addiction 90 (12), pp. 1635–45.
Klee, H., Faugier, J., Hayes, C. and Morris, J. (1991), ‘The sharing of injecting equipment among drug users attending prescribing clinics and those using needle-exchanges’, British Journal of Addiction 86, pp. 217–23.
Kwiatkowski, C. F. and Booth, R. E. (2001), ‘Methadone maintenance as HIV risk reduction with street-recruited injecting drug users’, Journal of Acquired Immune Deficiency Syndromes 26 (5), pp. 483–9.
Lamden, K. H., Kennedy, N., Beeching, N. J., et al. (1998), ‘Hepatitis B and hepatitis C virus infections: risk factors among drug users in northwest England’, Journal of Infection 37 (3), pp. 260–9.
Linssen, L., de Jong, W. and Wolf, J. (2000), Gebruiksruimten: Een systematisch overzicht van de voorziening en de effecten ervan, Trimbos-Instituut, ontwikkelcentrum Social Verslavingsbeleid, Utrecht.
Ljungberg, B., Christensson, B., Tunving, K., et al. (1991), ‘HIV prevention among injecting drug users: three years of experience from a syringe exchange program in Sweden’, Journal of Acquired Immune Deficiency Syndromes 4 (9), pp. 890–5.
Longshore, D., Hsieh, S., Danila, B. and Anglin, M. D. (1993), ‘Methadone maintenance and syringe sharing’, International Journal of the Addictions 29, pp. 983–96.
Longshore, D., Bluthenthal, R. N. and Stein, M. D. (2001), ‘Needle exchange program attendance and injection risk in Providence, Rhode Island’, AIDS Education and Prevention 13 (1), pp. 78–90.
Lott, D., Strain, E. C., Brooner, R., Bigelow, G. and Johnson, R. (2006), ‘HIV risk behaviours during pharmacologic treatment for opioid dependence: a comparison of levomethadyl acetate hydrochloride, buprenorphine, and methadone’, Journal of Substance Abuse Treatment 31 (2), pp. 187–94.
Lurie, P. (1997), ‘Invited commentary: le mystere de Montreal’, American Journal of Epidemiology 146 (12), pp. 1003–6; discussion pp. 1007–10.
McAuley, A., Lindsay, G., Woods, M. and Louttit, D. (2009), ‘Responsible management and use of a personal take-home naloxone supply: a pilot project’, Drugs: Education Prevention and Policy, DOI: 10.1080/09687630802530712.
MacDonald, M. A., Wodak, A. D., Dolan, K. A., et al. (2000), ‘Hepatitis C virus antibody prevalence among injecting drug users at selected needle and syringe programs in Australia, 1995–1997: collaboration of Australian NSPs’, Medical Journal of Australia 172 (2), pp. 57–61.
MacDonald, M., Law, M. G., Kaldor, J. M., Hales, J. and Dore, G. J. (2003), ‘Effectiveness of needle and syringe programmes for preventing HIV transmission’, International Journal of Drug Policy 14 (5–6), pp. 353–7.
McGregor, C., Ali, R., Lokan, R., Christie, P. and Darke, S. (2002), ‘Accidental fatalities among heroin users in South Australia, 1994–1997: toxicological findings and circumstances of death’, Addiction Research and Theory, pp. 335–46.
Magura, S., Siddiqui, Q., Shapiro, J., et al. (1991), ‘Outcomes of an AIDS prevention program for methadone patients’, International Journal of the Addictions 26 (6), pp. 629–55.
Magura, S., Nwakeze, P. and Demsky, S. (1998), ‘Pre-and in-treatment predictors of retention in methadone treatment using survival analysis’, Addiction 93 (1), pp. 51–60.
Maher, L., Jalaludin, B., Chant, K. G., et al. (2006), ‘Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia’, Addiction 101 (10), pp. 1499–508.
Mansson, A. S., Moestrup, T., Nordenfelt, E. and Widell, A. (2000), ‘Continued transmission of hepatitis B and C viruses, but no transmission of human immunodeficiency virus among intravenous drug users participating in a syringe/needle exchange program’, Scandinavian Journal of Infectious Diseases 32 (3), pp. 253–8.
Margolin, A., Avants, S. K., Warburton, L. A., Hawkins, K. A. and Shi, J. (2003), ‘A randomized clinical trial of a manual-guided risk reduction intervention for HIV-positive injection drug users’, Health Psychology 22 (2), pp. 223–8.
Mark, H. D., Nanda, J., Davis-Vogel, A., et al. (2006), ‘Profiles of self-reported HIV-risk behaviours among injection drug users in methadone maintenance treatment, detoxification, and needle exchange programs’, Public Health Nursing 23 (1), pp. 11–19.
Marsch, L. A., Bickel, W. K., Badger, G. J. and Jacobs, E. A. (2005), ‘Buprenorphine treatment for opioid dependence: the relative efficacy of daily, twice and thrice weekly dosing’, Drug and Alcohol Dependence 77 (2), pp. 195–204.
Mathers, B. M., Degenhardt, L., Wiessing, L., et al. (2008), ‘Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review’, Lancet 372 (9651), pp. 1733–45.
Maxwell, S., Bigg, D., Stanczykiewicz, K. and Carlberg-Racich, S. (2006), ‘Prescribing naloxone to actively injecting heroin users: a program to reduce heroin overdose deaths’, Journal of Addictive Diseases 25 (3), pp. 89–96.
Meandzija, B., O’Connor, P. G., Fitzgerald, B., Rounsaville, B. J. and Kosten, T. R. (1994), ‘HIV infection and cocaine use in methadone maintained and untreated intravenous drug users’, Drug and Alcohol Dependence 36 (2), pp. 109–13.
Meijer, G., de Jong, A., Koeter, M. and Bieleman, B. (2001), Gebruik van de straat: Evaluatie gebruiksruimte binnenstad-Zuid Groningen, INTRAVAL, Groningen-Rotterdam.
Metzger, D. S., Woody, G. E., McLellan, A. T., et al. (1993), ‘Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: an 18-month prospective follow-up’, Journal of Acquired Immune Deficiency Syndromes 6 (9), pp. 1049–56.
Meyeno, C., Zulaica, D. and Parras, F. (2000), ‘Prisons: needle exchange programmes in prisons in Spain’, Canadian HIV/AIDS Policy & Law Newsletter 5, pp. 20–21.
Miller, C. L., Wood, E., Spittal, P. M., et al. (2004), ‘The future face of coinfection: prevalence and incidence of HIV and hepatitis C virus coinfection among young injection drug users’, Journal of Acquired Immune Deficiency Syndromes 36 (2), pp. 743–9.
Milloy, M. and Wood, E. (2009), ‘Emerging role of supervised injecting facilities in human immunodeficiency virus prevention’, Addiction 104 (4), pp. 620–1.
Millson, P., Myers, T., Calzavara, L., et al. (2003), ‘Regional variation in HIV prevalence and risk behaviours in Ontario injection drug users (IDU)’, Canadian Journal of Public Health 94, pp. 431–5.
Minder Nejedly, M. and Bürki, C. M. (1996), Monitoring HIV risk behaviours in a street agency with injection room in Switzerland, Medizinischen Fakultät, Universität Bern, Bern.
Moher, D., Schulz, K., Altman, D. and CONSORT Group (2001), ‘The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials’, Lancet 357 pp. 1191–4.
Monterroso, E. R., Hamburger, M. E., Vlahov, D., et al. (2000), ‘Prevention of HIV infection in street-recruited injection drug users: the Collaborative Injection Drug User Study (CIDUS)’, Journal of Acquired Immune Deficiency Syndromes 25 (1), pp. 63–70.
Moss, A. R., Vranizan, K., Gorter, R., et al. (1994), ‘HIV seroconversion in intravenous drug users in San Francisco, 1985–1990’, AIDS 8 (2), pp. 223–31.
MSIC Evaluation Committee (2003), Final report of the evaluation of the Sydney Medically Supervised Injecting Centre, University of New South Wales, Sydney.
National Treatment Agency (2009), ‘Life saving kits to be given to families of injecting drug users in groundbreaking scheme’. Available at http://www.nta.nhs.uk/media/media_releases/2009_media_releases/life_saving_kits_to_be_given_to_families_of_injecting_drug_users.aspx.
Nelles, J., Dobler-Mikola, A. and Kaufmann, B. (1997), ‘Provision of syringes and prescription of heroin in prison: the Swiss experience in the prisons of Hindelbank and Oberschongrun,’ in Nelles, J. and Fuhrer, A. (eds), Harm reduction in prison, Peter Lang, Bern, pp. 239–62.
Nelson, K. E., Vlahov, D., Cohn, S., et al. (1991), ‘Human immunodeficiency virus infection in diabetic intravenous drug users’, JAMA 266 (16), pp. 2259–61.
Novick, D. M., Joseph, H., Croxson, T. S., et al. (1990), ‘Absence of antibody to human immunodeficiency virus in long-term, socially rehabilitated methadone maintenance patients’, Archives of Internal Medicine 150, pp. 97–9.
Obadia, Y., Feronia, I., Perrin, V., Vlahov, D., and Moatti, J. P. (1999), ‘Syringe vending machines for injection drug users: an experiment in Marseille, France’, American Journal of Public Health 89 (12), pp. 1852–4.
Oliver, K. J., Maynard, H., Friedman, S. R. and Des Jarlais, D. C. (1994), ‘Behavioral and community impact of the Portland Syringe Exchange Program’, paper presented at the Proceedings of a Workshop on Needle Exchange and Bleach Distribution Programs, Washington, DC.
Ouellet, L., Huo, D. and Bailey, S. L. (2004), ‘HIV risk practices among needle exchange users and nonusers in Chicago’, Journal of Acquired Immune Deficiency Syndromes 37 (1), pp. 1187–96.
Palmateer, N., Kimber, J., Hickman, M., et al. (2008), Evidence for the effectiveness of harm reduction interventions in preventing hepatitis C transmission among injecting drug users, Prevention Working Groups of the Advisory Council on the Misuse of Drugs and the Hepatitis C Action Plan for Scotland, Glasgow.
Palmateer, N., Kimber, J., Hickman, M., et al. (2010), ‘Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and HIV transmission among injecting drug users: a review of reviews’, Addiction , DOI:10.1111/j.1360-0443.2009.02888.x.
Paone, D., Des Jarlais, D. C., Caloir, S., et al. (1994), ‘New York City syringe exchange: an overview’, paper presented at the Proceedings of a Workshop on Needle Exchange and Bleach Distribution Programs, Washington, DC.
Parmar, M. (2008), ‘NALoxone InVEstigation (N-ALIVE) pilot randomised controlled trial (RCT)’. Available at http://www.controlled-trials.com/ISRCTN34044390/.
Patrick, D. M., Strathdee, S. A., Archibald, C. P., et al. (1997), ‘Determinants of HIV seroconversion in injection drug users during a period of rising prevalence in Vancouver’, International Journal of STD & AIDS 8 (7), pp. 437–45.
Patrick, D. M., Tyndall, M. W., Cornelisse, P. G., et al. (2001), ‘Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection’, Canadian Medical Association Journal 165 (7), pp. 889–95.
Peak, A., Rana, S., Maharjan, S. H., Jolley, D. and Crofts, N. (1995), ‘Declining risk for HIV among injecting drug users in Kathmandu, Nepal: the impact of a harm-reduction programme’, AIDS 9 (9), pp. 1067–70.
Petticrew, M. (2009), ‘Systematic reviews in public health: old chestnuts and new challenges’, Bulletin of the World Health Organization 87 (3), pp. 163–163A.
Piper, T. M., Stancliff, S., Rudenstine, S., et al. (2008), ‘Evaluation of a naloxone distribution and administration program in New York City’, Substance Use and Misuse 43 (7), pp. 858–70.
Poschadel, S., Höger, R., Schnitzler, J. and Schreckenberger, D. (2003), Evaluation der Arbeit der Drogenkonsumräume in der Bundesrepublik Deutschland: Endbericht im Auftrag des Bundesministeriums für Gesundheit, Nomos-Verlags-Gesellschaft, Baden-Baden.
Poser, W., Koc, J. and Ehrenreich, H. (1995), ‘Methadone maintenance treatment’, British Medical Journal 310, pp. 463.
Pouget, E. R., Deren, S., Fuller, C. M., et al. (2005), ‘Receptive syringe sharing among injection drug users in Harlem and the Bronx during the New York State Expanded Syringe Access Demonstration Program’, Journal of Acquired Immune Deficiency Syndromes 39 (4), pp. 471–7.
Power, R. and Nozhkina, N. (2002), ‘The value of process evaluation in sustaining HIV harm reduction in the Russian Federation’, AIDS 16 (2), pp. 303–04.
Reitox (2008), ‘National reports to the EMCDDA on new developments, trends and in-depth information on selected issues’, EMCDDA. Available at http://www.emcdda.europa.eu/publications/searchresults?action=list&ty pe=PUBLICATIONS&SERIES_PUB=w203.
Reyes Fuentes, V. C. (2003), 15 Jahre Fixerraum Bern. Auswirkungen auf soziale und medizinische Aspekte bei Drogenabhängigen, University of Bern, Bern.
Rezza, G., Sagliocca, L., Zaccarelli, M., et al. (1996), ‘Incidence rate and risk factors for HCV seroconversion among injecting drug users in an area with low HIV seroprevalence’, Scandinavian Journal of Infectious Diseases 28 (1), pp. 27–9.
Rhoades, H., Creson, D., Elk, R., Schmitz, J. and Grabowski, J. (1998), ‘Retention, HIV risk, and illicit drug use during treatment: methadone dose and visit frequency’, American Journal of Public Health 88, pp. 34–9.
Richard, A. J., Mosier, V. and Atkinson, J. S. (2002), ‘New syringe acquisition and multi-person use of syringes among illegal drug users’, Journal of Public Health Policy 23 (3), pp. 324–43.
Ronco, C., Spuhler, G., Coda, P. and Schopfer, R. (1996), ‘Evaluation der Gassenzimmer I, II und III in Basel’, Sozial und Praventivmedizin 41, pp. S58–S68.
Roy, E., Alary, M., Morissette, C., et al. (2007), ‘High hepatitis C virus prevalence and incidence among Canadian intravenous drug users’, International Journal of STD and AIDS 18 (1), pp. 23–7.
Sackett, D. L., Rosenberg, J., Muir Gray, J. A., Haynes, R. B. and Richardson, W. S. (1996), ‘Evidence based medicine: what it is and what it isn’t’, British Medical Journal 312, pp. 71–2.
Sarkar, K., Mitra, S., Bal, B., Chakraborty, S. and Bhattacharya, S. K. (2003), ‘Rapid spread of hepatitis C and needle exchange programme in Kolkata, India’, Lancet 361 (9365), pp. 1301–2.
Saxon, A., Calsyn, D. and Jackson, T. (1994), ‘Longitudinal changes in injection behaviors in a cohort of injection drug users’, Addiction 89, pp. 191–202.
Schechter, M. T., Strathdee, S. A., Cornelisse, P. G., et al. (1999), ‘Do needle exchange programmes increase the spread of HIV among injection drug users? An investigation of the Vancouver outbreak’, AIDS 13 (6), pp. F45–51.
Schoenbaum, E. E., Hartel, D. M. and Gourevitch, M. N. (1996), ‘Needle exchange use among a cohort of injecting drug users’, AIDS 10 (14), pp. 1729–34.
Schroeder, J., Epstein, D., Umbricht, A. and Preston, K. (2006), ‘Changes in HIV risk behaviours among patients receiving combined pharmacological and behavioral interventions for heroin and cocaine dependence’, Addictive Behaviors 31 (5), pp. 868–79.
Seal, K. H., Thawley, R., Gee, L., et al. (2005), ‘Naloxone distribution and cardiopulmonary resuscitation training for injection drug users to prevent heroin overdose death: a pilot intervention study’, Journal of Urban Health 82 (2), pp. 303–11.
Sees, K. L., Delucchi, K. L., Masson, C., et al. (2000), ‘Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial’, JAMA 283 (10), pp. 1303–10.
Selvey, L. A., Denton, M. and Plant, A. J. (1997), ‘Incidence and prevalence of hepatitis C among clients of a Brisbane methadone clinic: factors influencing hepatitis C serostatus’, Australian and New Zealand Journal of Public Health 21 (1), pp. 102–04.
Serpelloni, G., Carrieri, M. P., Rezza, G., et al. (1994), ‘Methadone treatment as a determinant of HIV risk reduction among injecting drug users: a nested case-control study’, AIDS Care 6 (2), pp. 215–20.
Sherman, S. G., Gann, D. S., Tobin, K. E., et al. (2009), ‘“The life they save may be mine”: diffusion of overdose prevention information from a city sponsored programme’, International Journal of Drug Policy 20 (2), pp. 137–42.
Shore, R., Marmor, M., Titus, S. and Des Jarlais, D. (1996), ‘Methadone maintenance and other factors associated with intraindividual temporal trends in injection drug use’, Journal of Substance Abuse Treatment 13, pp. 241–8.
Simpson, D. D., Joe, G. W., Rowan-Szal, G. and Greener, J. (1995), ‘Client engagement and change during drug abuse treatment’, Journal of Substance Abuse 7 (1), pp. 117–34.
Singer, M., Himmelgreen, D., Weeks, M. R., Radda, K. E. and Martinez, R. (1997), ‘Changing the environment of AIDS risk: findings on syringe exchange and pharmacy sales of syringes in Hartford, CT’, Medical Anthropology 18 (1), pp. 107–30.
Small, D. (2007), ‘Fools rush in where angels fear to tread: playing God with Vancouver’s supervised injection facility in the political borderland’, International Journal of Drug Policy 18 (1), pp. 18–26.
Smyth, B. P., Keenan, E. and O’Connor, J. J. (1999), ‘Evaluation of the impact of Dublin’s expanded harm reduction programme on prevalence of hepatitis C among short-term injecting drug users’, Journal of Epidemiology and Community Health 53 (7), pp. 434–5.
Somaini, B., Wang, J. and Persozo, M. (2000), ‘A continuing concern: HIV and hepatitis testing and prevalence among drug users in substitution programmes in Switzerland’, AIDS Care 12, pp. 449–60.
Sorensen, J. L. and Copeland, A. L. (2000), ‘Drug abuse treatment as an HIV prevention strategy: a review’, Drug and Alcohol Dependence 59 (1), pp. 17–31.
Sporer, K. A. (2003), ‘Strategies for preventing heroin overdose’, British Medical Journal 326 (7386), pp. 442–44.
Stallwitz, A. and Stöver, H. (2007), ‘The impact of substitution treatment in prisons: a literature review’, International Journal Drug Policy 18 (6), pp. 464–74.
Stark, K., Herrmann, U., Ehrhardt, S. and Bienzle, U. (2006), ‘A syringe exchange programme in prison as prevention strategy against HIV infection and hepatitis B and C in Berlin, Germany’, Epidemiology and Infection 134 (4), pp. 814–19.
Stark, K., Muller, R., Bienzle, U. and Guggenmoos-Holzmann, I. (1996), ‘Methadone maintenance treatment and HIV risk-taking behaviour among injecting drug users in Berlin’, Journal of Epidemiology and Community Health 50 (5), pp. 534–7.
Stevens, A., Stöver, H. and Brentari, C. (2010), ‘Criminal justice approaches to harm reduction in Europe’, in European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), Harm reduction: evidence, impacts and challenges, Rhodes, T. and Hedrich, D. (eds), Scientific Monograph Series No. 10, Publications Office of the European Union, Luxembourg.
Stoltz, J. A., Wood, E., Small, W., et al. (2007), ‘Changes in injecting practices associated with the use of a medically supervised safer injection facility’, Journal of Public Health (Oxford) 29 (1), pp. 35–9.
Stöver, H. and Nelles, J. (2003), ‘Ten years of experience with needle and syringe exchange programmes in European prisons’, International Journal of Drug Policy 14 (5–6), pp. 437–44.
Strang, J., Marsden, J., Cummins, M., et al. (2000), ‘Randomized trial of supervised injectable versus oral methadone maintenance: report of feasibility and 6-month outcome’, Addiction 95 (11), pp. 1631–45.
Strang, J., Manning, V., Mayet, S., et al. (2008), ‘Overdose training and take-home naloxone for opiate users: prospective cohort study of impact on knowledge and attitudes and subsequent management of overdoses’, Addiction 103 (10), pp. 1648–57.
Strathdee, S. A., Patrick, D. M., Currie, S. L., et al. (1997), ‘Needle exchange is not enough: lessons from the Vancouver injecting drug use study’, AIDS 11 (8), pp. F59–65.
Taylor, A., Goldberg, D., Hutchinson, S., et al. (2000), ‘Prevalence of hepatitis C virus infection among injecting drug users in Glasgow 1990–1996: are current harm reduction strategies working?’ Journal of Infection 40 (2), pp. 176–83.
Teeson, M., Ross, J., Darke, S., et al. (2006), ‘One year outcomes for heroin dependence: findings from the Australian Treatment Outcome Study (ATOS)’, Drug and Alcohol Dependence 83 (2), pp. 174–80.
Thiede, H., Hagan, H. and Murrill, C. S. (2000), ‘Methadone treatment and HIV and hepatitis B and C risk reduction among injectors in the Seattle area’, Journal of Urban Health 77 (3), pp. 331–45.
Tilson, H., Aramrattana, A., Bozzette, S., et al. (2007), ‘Preventing HIV infection among injecting drug users in high risk countries: an assessment of the evidence’, Institute of Medicine, Washington, DC.
Tobin, K. E., Sherman, S., Beilenson, P., Welsh, C. and Latkin, C. A. (2009), ‘Evaluation of the Staying Alive programme: training injection drug users to properly administer naloxone and save lives’, International Journal of Drug Policy 20 (2), pp. 131–6.
Valente, T., Foreman, R. K., Junge, B. and Vlahov, D. (2001), ‘Needle-exchange participation effectiveness and policy: syringe relay, gender, and the paradox of public health’, Journal of Urban Health 78, pp. 340–9.
van Ameijden, E. J. and Coutinho, R. A. (1998), ‘Maximum impact of HIV prevention measures targeted at injecting drug users’, AIDS 12 (6), pp. 625–33.
van Ameijden, E. J., van den Hoek, J. A., van Haastrecht, H. J. and Coutinho, R. A. (1992), ‘The harm reduction approach and risk factors for human immunodeficiency virus (HIV) seroconversion in injecting drug users, Amsterdam’, American Journal of Epidemiology 136 (2), pp. 236–43.
van Ameijden, E. J., van den Hoek, J. A., Mientjes, G. H. and Coutinho, R. A. (1993), ‘A longitudinal study on the incidence and transmission patterns of HIV, HBV and HCV infection among drug users in Amsterdam’, European Journal of Epidemiology 9 (3), pp. 255–62.
van Ameijden, E. J., van den Hoek, A. R. and Coutinho, R. A. (1994), ‘Injecting risk behavior among drug users in Amsterdam, 1986 to 1992, and its relationship to AIDS prevention programs’, American Journal of Public Health 84 (2), pp. 275–81.
van Ameijden, E. J., Langendam, M. W. and Coutinho, R. A. (1999), ‘Dose-effect relationship between overdose mortality and prescribed methadone dosage in low-threshold maintenance programs’, Addictive Behaviours 24 (4), pp. 559–63.
van den Berg, C., Smit, C., van Brussel, G., Coutinho, R. A. and Prins, M. (2007), ‘Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users’, Addiction 102, pp. 1454–62.
van den Hoek, J. A., van Haastrecht, H. J. and Coutinho, R. A. (1989), ‘Risk reduction among intravenous drug users in Amsterdam under the influence of AIDS’, American Journal of Public Health 79 (10), pp. 1355–7.
van der Poel, A., Barendregt, C. and van de Mheen, D. (2003), ‘Drug consumption rooms in Rotterdam: an explorative description’, European Addiction Research 9, pp. 94–100.
Vazirian, M., Nassirimanesh, B., Zamani, S., et al. (2005), ‘Needle and syringe sharing practices of injecting drug users participating in an outreach HIV prevention program in Tehran, Iran: a cross-sectional study’, Harm Reduction Journal 2, p. 19.
Vertefeuille, J., Marx, M. A., Waimar, T., et al. (2000), ‘Decline in self-reported high-risk injection-related behaviors among HIV-seropositive participants in the Baltimore needle exchange program’, AIDS and Behavior 4 (4), pp. 381–8.
Vickerman, P., Hickman, M., Rhodes, T. and Watts, C. (2006), ‘Model projections on the required coverage of syringe distribution to prevent HIV epidemics among injecting drug users’, Journal of Acquired Immune Deficiency Syndromes 42 (3), pp. 355–61.
Villaneuva, M. G. (2002), ‘Programa de Intercambia de Jeringuillas en el Centre Penitenciario de Pamplona’, Rev Esp Sanid Penit 4, pp. 18–23.
Vlahov, D., Junge, B., Brookmeyer, R., et al. (1997), ‘Reductions in high-risk drug use behaviors among participants in the Baltimore needle exchange program’, Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 16 (5), pp. 400–06.
Wagner, K., Valente, T., Casanova, M., et al. (in press), ‘Evaluation of an overdose prevention and response training programme for injection drug users in the Skid Row area of Los Angeles, CA’, International Journal of Drug Policy, DOI: 10.1016/j.drugpo.2009.01.003.
Ward, J., Mattick, R. P. and Hall, W. (1997), Methadone maintenance treatment and other opioid replacement therapies, Harwood Academic Press, Amsterdam.
Watters, J. (1994), ‘Syringe and needle exchange as HIV/AIDS prevention for injecting drug users’, Journal of the American Medical Association 271, pp. 115–20.
WHO (World Health Organization) (2007), Guide to starting and managing needle and syringe programmes, World Health Organization, Geneva.
WHO, UNAIDS (Joint United Nations Programme on HIV/AIDS) and UNODC (United Nations Office on Drugs and Crime) (2007), Effectiveness of interventions to manage HIV in prisons: opioid substitution therapies and other drug dependence treatment, WHO, Geneva.
Williams, A. B., McNelly, E. A., Williams, A. E. and D’Aquila, R. T. (1992), ‘Methadone maintenance treatment and HIV type 1 seroconversion among injecting drug users’, AIDS Care 4 (1), pp. 35–41.
Wodak, A. and Cooney, A. (2004), Effectiveness of sterile needle and syringe programming in reducing HIV/AIDS among injecting drug users, WHO, Geneva.
Wodak, A. and Cooney, A. (2006), ‘Do needle syringe programs reduce HIV infection among injecting drug users? A comprehensive review of the international evidence’, Substance Use and Misuse 41 (6–7), pp. 777–813.
Wood, E., Tyndall, M. W., Spittal, P. M., et al. (2002), ‘Factors associated with persistent high-risk syringe sharing in the presence of an established needle exchange programme’, AIDS 16 (6), pp. 941–3.
Wood, E., Kerr, T., Spittal, P. M., et al. (2003), ‘An external evaluation of a peer-run “unsanctioned” syringe exchange program’, Journal of Urban Health 80 (3), pp. 455–64.
Wood, E., Tyndall, M., Stoltz, J., et al. (2005), ‘Factors associated with syringe sharing among users of a medically supervised safer injecting facility’, American Journal of Infectious Diseases 1 (1), pp. 50–4.
Wood, E., Tyndall, M., Montaner, J., and Kerr, T. (2006), ‘Summary of findings from the evaluation of a pilot medically supervised safer injecting facility’, Canadian Medical Association Journal 175 (11), pp. 1399–404.
Wright, N. M. J. and Tompkins, C. N. E. (2006), ‘A review of the evidence for the effectiveness of primary prevention interventions for hepatitis C among injecting drug users’, Harm Reduction Journal 3 (27), DOI: 10.1186/1477-7517-3-27.
Yancovitz, S., Des Jarlais, D., Peskoe-Peyser, N., et al. (1991), ‘A randomised trial of an interim methadone maintenance clinic’, American Journal of Public Health 81, pp. 1185–91.
Zador, D., Sunjic, S. and Darke, S. (1996), ‘Heroin related deaths in New South Wales in 1992: toxicological findings and circumstances’, Medical Journal of Australia 164, pp. 204–07.
Zurhold, H., Kreuzfeld, N., Degwitz, P. and Verthein, U. (2001), Drogenkonsumräume: Gesundheitsförderung und Minderung öffentlicher Belastungen in europäischen Grossstädten, Lambertus, Freiburg.
| < Prev | Next > |
|---|












