Review on clinical studies with cannabis and cannabinoids 2005-2009
| Articles - Cannabis, marijuana & hashisch |
Drug Abuse
Review on clinical studies with cannabis and cannabinoids 2005-2009
Arno Hazekamp, Franjo Grotenhermen
Institute Biology Leiden, Leiden University, The Netherlands
nova-Institut, Chemiepark Knapsack, Industriestralle, D-50354 Hürth, Germany
Abstract
To date, a large number of controlled clinical trials have been done evaluating the therapeutic applications of cannabis and cannabis-based preparations. In 2006, an excellent review was published, discussing the clinical trials performed in the period 1975 to June 2005 [Ben Amar 2006]. The current review reports on the more recent clinical data available. A systematic search was performed in the scientific database of PubMed, focused on clinical studies that were randomized, (double) blinded, and placebo-controlled. The period screened was from July 1, 2005 up to August 1,2009.
The key words used were: cannabis, marijuana, marihuana, hashish, cannabinoid(s), tetrahydrocannabinol, THC, CBD, dronabinol, Marinol, nabilone, Cannador and Sativex.
For the final selection, only properly controlled clinical trials were retained. Open-label studies were excluded, except if they were a direct continuation of a study discussed here. Thirty-seven controlled studies evaluating the therapeutic effects of cannabinoids were identified. For each clinical trial, the country where the project was held, the number of patients assessed, the type of study and comparisons done, the products and the dosages used, their efficacy and their adverse effects are described. Based on the clinical results, cannabinoids present an interesting therapeutic potential mainly as analgesics in chronic neuropathic pain, appetite stimulants in debilitating diseases (cancer and AIDS), as well as in the treatment of multiple sclerosis.
Keywords: cannabinoids, cannabis, therapeutic potential, controlled clinical trial, efficacy, safety
This article can be downloaded, printed and distributed freely for any non-commercial purposes, provided the original work is properly cited (see copyright info below). Available online at www.cannabis-med org
Author's address: Arno Hazekamp, This e-mail address is being protected from spambots. You need JavaScript enabled to view it
Introduction and Method
There is a growing number of clinical studies that indicate that cannabis or single cannabinoids may have medicinal value for certain diseases and under certain conditions. In the period from 1975 to current, at least 110 controlled clinical studies have been published, assessing well over 6100 patients suffering from a wide range of illnesses. Also the mechanisms of action are becoming increasingly clear since the discovery of the endocannabinoid system and its physiological functions. In 2006, the Canadian researcher Ben Amar published a review discussing the results of clinical trials performed with cannabis and cannabinoids over the period 1975 to June 2005. The review presented here reports on the period following this, discussing the clinical trials published since then. Together, these two reviews can provide a convenient overview of clinical studies over the last 34 years. The methodology of this review has been adopted from Ben Amar [2006].
In order to assess the current knowledge on the therapeutic potential of Cannabis, phytocannabinoids, and medicinal preparations directly based on phyto-cannabinoids, a systematic search was performed in the scientific database of PubMed. Hosted by the U.S. National Library of Medicine, this database contains about 20 million scientific publications from the field of life sciences and biomedical information. The period screened was from July 1, 2005 up to August 1, 2009. Clinical data from the period up to July 2005 has been previously reviewed by Ben Amar [2006]. The search focused on clinical studies that were randomized, (double) blinded, and placebo-controlled. The key words used were: cannabis, maruana, marihuana, hashish, cannabinoid(s), tetrahydrocannabinol, THC, CBD, dronabinol, Marinol, nabilone, Cannador and Sativex. After initial sorting, all articles and reviews including clinical protocols or a summary of the literature evaluating the therapeutic potential of cannabinoids in humans were read. For the final selection, only properly controlled clinical trials were retained, thus open-label studies were excluded, except when they were a direct continuation of a clinical trial discussed in this paper.
The research included the works and data available in English, but also other languages (2x German, lx Danish). A range of different cannabis-based products are described in the studies presented in this review. For the ease of the less experienced reader, these preparations are briefly discussed below:
Cannabis refers to the dried flowertops of the female plant of Cannabis. This herbal product is also commonly known as marijuana or marihuana. The main way to administer cannabis is by smoking, which is also the way most medicinal users consume it. For clinical trials, most often these materials are standardized for their content (in % of dry weight) of THC. THC, or delta-9-tetrahydrocannabinol, is the pharmacologically and toxicologically most relevant constituent found in the Cannabis plant, producing a myriad of effects in animals and humans. The most well-established palliative effect of THC is the inhibition of chemotherapy-induced nausea and vomiting, mainly in cancer patients.Pure THC can be derived from natural sources (extraction from cannabis plants) or produced synthetically. Chemically, THC belongs to a group of closely related compounds known as cannabinoids, and they are commonly considered the main bioactive components of Cannabis.
Up to date, more than 100 different cannabinoids have been described, but only a few of the major ones have been characterized for biological activities, including cannabidiol (CBD, see below) and cannabinol (CBN).
Dronabinol is the INN (international non-proprietary name) of the isomer of delta-9-tetrahydrocannabinol that is present in the cannabis plant, the (-)-trans-isomer. This is the only naturally occurring of the four isomers. Oral capsules containing synthetically manufactured dronabinol are available under the name Marinol (see below).
CBD, or cannabidiol, is the major non-psychotropic cannabinoid found in Cannabis. It has shown anti-epileptic, anti-inflammatory, anti-emetic, muscle relaxing, anxiolytic, neuroprotective and anti-psychotic activity and reduces the psychoactive effects of THC [Russo 2006]. The mode of action of cannabidiol is not fully understood and several mechanisms have been proposed: (1) CBD acts as antagonist at the central CB, receptor and was able to inhibit several CB, mediated THC effects [Zuardi et al. 1982]. In a study by Petitet et al. (1998), CBD considerably reduced the receptor activation by the potent classical CB, receptor agonist CP55940. (2) CBD stimulates the vanilloid receptor type 1 (VR1) with a maximum effect similar in efficacy to that of capsaicin [Bisogno et al. 2001]. (3) CBD inhibits the uptake and hydrolysis of the endocannabinoid anandamide, thus increasing its concentration [Bisogno et al. 2001, Mechoulam & Hanus 2002]. (4) Finally, CBD may also increase the plasma THC level [Bornheim et al. 1995] by inhibiting hepatic microsomal THC metabolism through inactivation of the cytochrome P-450 oxidative system [Bornheim et al. 1998, Jaeger et al. 1996]. However, there was no or minimal effect of CBD on plasma levels of THC in man [Agurell et al. 1981, Hunt et al. 1981]. Further mechanisms have been described.
Marinol® (Solvay Pharmaceuticals, Belgium) is a synthetic version of dronabinol. It is formulated as a capsule containing synthetic dronabinol in sesame oil. In the US it is indicated for the treatment of anorexia associated with weight loss in patients with AIDS and nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments. The patent on Marinol will expire in 2011, opening the way for the development of generic preparations of synthetic, as well as naturally-derived, THC.
Nabilone (Valeant Pharmaceuticals International, USA) is a synthetic analogue of THC which binds to the cannabinoid C131 receptor. In Canada, the United States, the United Kingdom and Mexico, nabilone is marketed as Cesamet®. It is registered for treatment of chemotherapy-induced nausea and vomiting that has not responded to conventional antiemetics. It is also used for other medical conditions.
Sativex® (GW Pharmaceuticals, UK) is a cannabisbased pharmaceutical product containing delta 9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in a 1:1 ratio, delivered in an oromucosal (into the mouth) spray. Because of the use of whole extracts, non-standardized amounts of ballast components are also present, such as minor cannabinoids and terpenoids. Sativex has been approved in Canada as adjunctive treatment for neuropathic pain in adults with multiple sclerosis (MS) and in cancer pain. Registration is pending in several European countries.
Cannador® (Society for Clinical Research, Germany) is an oral capsule containing a whole plant extract, with standardized THC content and a CBD amount controlled to lie within a fixed narrow range with a THC:CBD ratio of about 2:1. It has been used in several clinical trials. It has been clinically tested for reduction of muscle stiffliess, spasms and associated pain in Multiple Sclerosis, for cachexia in cancer patients and for post-operative pain management.
Results
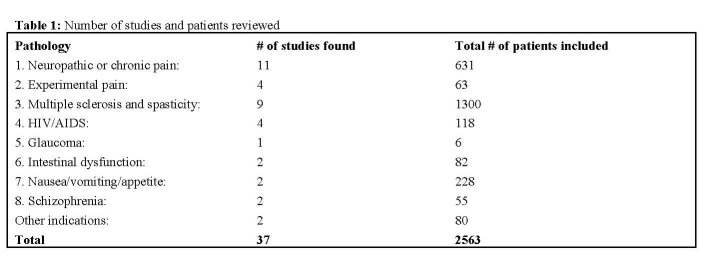
The review identified 8 main pathologies in which controlled studies on cannabinoids have been published: they are listed below. A number of other illnesses have been grouped under 'other indications'. Although experimentally induced pain is obviously not a pathological condition, it has been included in this review because it may add to our understanding of the use of cannabis for pain control.
In total, 37 controlled studies evaluating the therapeutic effects of cannabis or cannabinoids were identified. For each clinical trial, the country where the project was held, the number of patients assessed the type of study and comparisons done, the products and the dosages used, and their efficacy are described. Noteworthy adverse and side effects for each study are discussed in the text.
Summary of the clinical trials
Neuropathic, chronic and acute pain
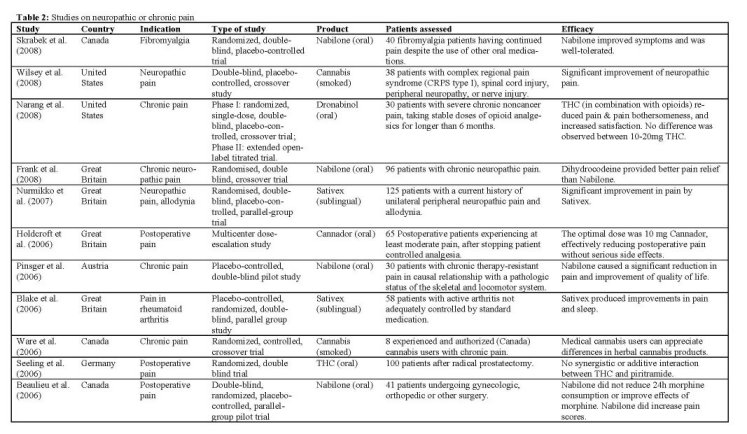
A range of studies has been done to determine the effect of nabilone on different types of pain. Based on the analgesic effects of cannabinoids in animal studies, it was hypothesized that nabilone would decrease morphine consumption, pain scores, nausea and vomiting following major surgery. IBeaulieu 2006] tested this hypothesis in a double-blind, randomized, placebocontrolled, parallel-group pilot trial with three doses of 1 or 2 mg of nabilone in the 24 hours after different types of major surgery. Surprisingly, and contrary to the main hypothesis, pain scores at rest and on movement were actually significantly higher in the 2 mg nabilone group compared to the other groups. Also, nabilone administration was not associated with a decrease in morphine consumption in patients. The most common adverse effects of nabilone were dry mouth, nausea and vomiting, respiratory depression, sedation and pruritus. No serious adverse events were observed. It is concluded from animal experiments that cannabinoid receptor and mu-opioid receptor agonists act synergistically with respect to antinociception. In order to demonstrate this effect under clinical conditions, a
study was performed with oral THC on patients after radical prostatectomy Seeling 2006]. It was expected that patients receiving THC required significantly less of the synthetic opioid analgesic piritramide to control their pain compared to patients on placebo. From the evening before the operation until the morning of the second postoperative day, patients received eight oral doses of either placebo or 5 mg THC, which is a significant amount of THC for any clinical trial. However, neither synergistic effect nor even an additive antinociceptive interaction with the combination of THC and piritramide was found, even though plasma concentrations of THC were measurable in all patients in the verum group.
In another study on postoperative pain, Holdcroft et al. 12006] aimed to investigate whether a single oral dose of Cannador could provide pain relief with minimal side effects. Sixty-five patients received a single dose of 5, 10, or 15 mg Cannador when they had at least moderate pain after stopping patient-controlled analgesia. Pain relief, pain intensity, and side effects were recorded over 6h after administration. Rescue analgesia was requested by all 11 patients (100%) receiving S mg, 15 of 30 patients (50%) receiving 10 mg, and 6 of 24 patients (25%) receiving 15 mg Cannador. There was a significant dose-response effect for decreasing pain intensity at rest, and increasing sedation. The number needed to treat (NINT) to prevent one rescue analgesia request for the 10-mg and 15-mg doses, relative to 5 mg, were 2.0 and 1.3, respectively, which is equivalent to many routinely used analgesics. The majority of adverse events affected the central nervous (14 of 26) or cardiovascular (6 of 26) systems, but none persisted after the study. The study was terminated because of a serious vasovagal adverse event in one patient receiving 15 mg.
In a study with nabilone, focusing on chronic pain, results were more promising. [Pinsger 2006] investigated the effect of an add-on treatment with nabilone on patients with chronic therapy-resistant pain in causal relationship with a pathologic status of the skeletal and locomotor system. From the results, it was obvious that the nabilone treatment (up to 1 mg per day) was superior, resulting in a decrease in several different pain-parameters (VAS), and an increase in quality of life (AQOL score). Although typical side effects of nabilone were commonly observed, such as dizziness, fatigue, dry mouth and sleepiness, the study concluded that a majority of patients classified nabilone intake in addition to the standard treatment as a positive measure. Thus, this kind of treatment may be an interesting and attractive enrichment of analgesic therapy.
Also Frank et aL 12008] focused on the potential analgesic effects of nabilone in neuropathic pain. Objective of this study was to compare the analgesic efficacy and side effects of this synthetic cannabinoid with those of the weak opioid dihydrocodeine for chronic neuropathic pain in 96 patients aged 23-84 years. It was found that the opioid was a better analgesic than nabilone. However, the clinical significance of the difference was small, and in fact the majority of patients had no clinically relevant drop in their pain score on either treatment. Nabilone was associated with more sickness than dihydrocodeine, while dihydrocodeine was associated with more tiredness and nightmares. No major adverse events occurred with either drug and both drugs were equally well tolerated. Although a dose of only 2 mg of nabilone was used in this study, the observed side effect profile argues against giving higher doses of the drug.
In patients with fibromyalgia, the first randomized, controlled trial to assess the benefit of nabilone on pain reduction and quality of life improvement was done only recently ISkrabek 2008]. It has been suggested that a clinical endocannabinoid deficiency may be involved in the etiology of fibromyalgia. As no treatment has been specifically approved for management of this condition, further research into treatment strategies is important. Nabilone (up to 1 mg BID) appeared to be a beneficial, well-tolerated treatment option for fibromyalgia patients, with significant benefits in pain relief and functional improvement. The most common side effects reported by subjects in the nabilone group included drowsiness (7/15), dry mouth (5/15), vertigo (4/15), and ataxia (3/15). No serious adverse events occurred during the study. There was a significant, but transient, increase in the weight of subjects treated with nabilone over the 8 weeks of the trial (mean 1.13 kg). Nabilone did not appear to have any lasting benefit in subjects when treatment was discontinued. During the study, subjects were asked to continue any current treatment for fibromyalgia, including breakthrough pain medications. Future studies could be done using nabilone as a single agent to determine its effect on pain and quality of life alone.
The efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy was assessed by INarang 2008] in a study combining a phase I (double-blind, single dose) and phase II (Open-label, multi-dose) trial. Results of the phase I study showed that patients who received dronabinol (10 or 20 mg) experienced decreased pain intensity and increased satisfaction compared with placebo. No differences in pain relief were found between the active treatments.
According to the authors, a lack of an active placebo may have contributed to unblinding. Phase II was an extended open-label titrated trial of dronabinol as addon medication to patients on stable doses of opioids. In this phase, titrated dronabinol contributed to significant relief of pain, reduced pain bothersomeness, and increased satisfaction compared with baseline. Overall, the use of dronabinol was found to result in additional analgesia among patients taking opioids for chronic noncancer pain. Subjects also showed improvements in quality of sleep. The most frequently reported side effects, compared to placebo, were dry mouth, tiredness, sleepiness, and drowsiness. Despite these side effects, subjects' overall satisfaction with treatment was significantly higher (54%) on active doses than placebo. The results imply that dronabinol may be a useful adjuvant analgesic for patients with persistent pain in spite of taking stable doses of opioids. Future studies need to examine whether the benefits and the side effects of THC among chronic pain patients change with prolonged use.
The majority of patients using cannabis for self-medication administer it by smoking, but there is currently no significant experience within the pharmaceutical world with the preparation and composition of cannabis cigarettes. As a result, it may be difficult to evaluate the experience of self-medicating patients, and to prove or disprove the medicinal effects of smoked cannabis. A unique study by [Ware 2006] addressed this issue by testing a range of different cannabis cigarettes in a randomized controlled crossover trial. Four different herbal cannabis preparations were tested among 8 experienced and authorized cannabis users with chronic pain. Preparations were varied with respect to grind size, THC content and humidity. The product with highest THC content (12%), highest humidity (14%) and largest grind size (10 mm) was rated highest overall. Significant differences were noted between preparations on overall appearance and color. While the small size of the study precludes broad conclusions, the study shows that medical cannabis users can appreciate differences in herbal product. A more acceptable cannabis product may increase recruitment and retention in clinical studies of medical cannabis.
IWilsey 2008] studied the effects of smoked cannabis on patients with central and peripheral neuropathic pain. A standardized procedure was used for smoking either high-dose (7%), low-dose (3.5%), or placebo cannabis. The amount of THC consumed was estimated to be 19 mg during the low-dose sessions and 34 mg during the high-dose sessions. Results indicated that cannabis may be effective at ameliorating neuropathic pain, and may be an alternative for patients who do not respond to, or cannot tolerate, other drugs. There was no apparent correlation of cannabinoid serum levels with analgesia. It was concluded that, as with opioids, cannabis does not rely on a relaxing or tranquilizing effect (e.g., anxiolysis) but rather reduces both the core component of nociception and the emotional aspect of the pain experience to an equal degree.
Undesirable consequences of smoking cannabis were clearly identifiable, but no participant dropped out because of an adverse event related to an experimental intervention.
In a first ever controlled trial of a cannabis preparation in rheumatoid arthritis, a significant analgesic effect was observed and disease activity was significantly suppressed following Sativex treatment [Blake 2006]. In comparison with placebo, a significant analgesic effect was observed and disease activity was significantly suppressed. Sativex produced statistically significant improvements in pain on movement, pain at rest, quality of sleep and inflammation (DAS28). The suppression of pain on movement, the primary endpoint, suggests a peripheral analgesic action, while the suppression of pain at rest may suggest a more central effect. The modest suppression of the present gold standard inflammation activity measure, the DAS28, might indicate an influence on the immune effector system. Importantly, the trial did not demonstrate significant toxicity and Sativex was generally well tolerated. The large majority of adverse effects were mild or moderate, and there were no adverse effect-related withdrawals or serious adverse effects in the active treatment group. About a quarter of patients receiving Sativex experienced transient dizziness at some point, though in all cases this was rated as mild.
A study by INurmikko 2007] demonstrated that Sativex is effective in the relief of peripheral neuropathic pain when given in addition to existing stable analgesia. A self-titrating regimen was used to optimise drug administration. Greater than 30% improvement in pain intensity, generally considered as clinically meaningful [Farrar 2000], was reported by 26% of subjects receiving Sativex, compared with 15% of patients taking placebo. A self-titration regimen permitted individual patients to optimize their dose on the basis of their own efficacy and tolerability response. Both experimental and human volunteer studies suggest that tolerance to some of the side effects of cannabis occurs within days of its repeated administration [Guy 2003, Jones 2002]. A self-titration regimen allows for this to occur, further optimizing the therapeutic response. An open-label extension study showed that the initial pain relief was maintained without dose escalation or toxicity for 52 weeks. The majority of patients took far less than the highest allowable dosage. Fifty-seven (91%) patients in the Sativex group experienced at least one adverse event (AE) during the course of the study compared with 48 (77%) patients in the placebo group. The AEs reported by the patients were mostly gastrointestinal, central nervous system related or topical. While reported gastrointestinal AEs were more common in the Sativex group, central nervous system AEs were not. Most were observed at onset of treatment, and in the majority described as mild. Intoxication scores remained low throughout the study. At recruitment, all patients were either non-responders to several conventional neuropathic analgesics, or were in severe pain despite taking appropriate therapy. Considering the re-
fractory nature of their pain, and that patients remained on their existing analgesia, the improvement of the ongoing pain in those on the active drug is encouraging.
Experimental pain
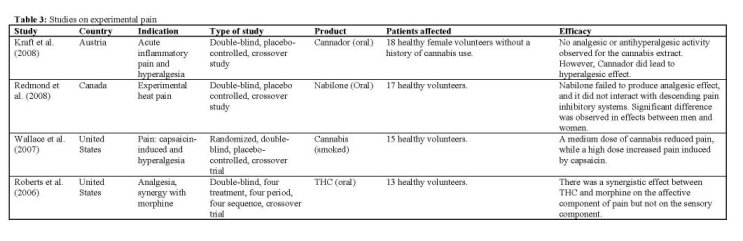
Co-administration of various cannabinoids with morphine has been found to produce a greater-than-additive effect with respect to antinociception in mice [Smith 1998], and crosstalk between the endocannabinoid- and endorphin-systems has been shown [Corchero 2004]. Therefore, the synergistic affective analgesic interaction between THC and morphine was determined in a double-blind, four treatment, crossover design IRoberts 2006]. Subjects received THC (5 mg orally) or placebo and 90 min later morphine (0.02 mg/kg) intravenously, or placebo. Fifteen minutes later subjects rated the pain associated with the application of thermal stimuli to skin. Neither morphine nor THC had a significant effect at the doses used, and there was no significant interaction between the two. A small, but non-significant synergy was found only for the affective component of pain. Subjects described a variety of mild euphoric or dysphoric effects, but no serious or unexpected toxicities occurred. The study concluded that future studies of THC or other cannabinoids in combination with opiates should focus upon clinical rather than experimental pain.
Based on the results of preclinical studies, another study IWallace 2007] hypothesized that inhaled cannabis would reduce capsaicin-induced pain and hyperalgesia, and change the affective quality of pain in a dose-dependent manner. In 19 healthy volunteers, the concentration-response effects were evaluated of low-, medium-, and high-dose smoked cannabis (respectively 2%, 4%, and 8% THC by weight). Only the medium dose cannabis significantly decreased capsaicin-induced pain. Interestingly, as has been observed in other studies [e.g. Kraft 2008], a significant increase in capsaicin-induced pain occurred with the high dose. The authors suggested that there is a window of modest analgesia for smoked cannabis, with lower doses decreasing pain and higher doses increasing it. There was a significant correlation between plasma levels of THC and metabolites with decrease in pain, but no correlation between the high-dose plasma levels and increase in pain. This suggests that there may be another compound within the cannabis used that was not measured but that was responsible for the increased pain at the high dose. Mild to moderate side effects were experienced by 7 of 19 subjects, primarily at the highest dose of cannabis, but no serious AEs occurred.
The double-blind, placebo-controlled, crossover study performed by Kraft et aL 12008] was designed to detect a potential analgesic activity of Cannador by two different and well-established human models of acute inflammatory pain and hyperalgesia. Only female volunteers were included, because animal studies using the same models have suggested a more pronounced effect of cannabinoids in females compared with males [Tseng 2004, Craft 2005]. The dose of THC in each cannabis administration was standardized to 20 mg. Also a significant amount of CBD was present (about 10 mg per administration). No analgesic or antihyperalgesic activity of this cannabis extract was found, even though the high levels of THC and its metabolites detected in the plasma of study subjects, and the occurrence of psychotropic side effects, argue for a sufficient bioavailability. In contrast, the results actually seem to support the impression that high doses of cannabinoids may cause hyperalgesia in certain acute pain conditions. One subject experienced acute psychotic symptoms after Cannador, but all symptoms spontaneously disappeared after 4 hours. Despite the standardized conditions, a broad variability in peak plasma levels for all measured cannabinoids was observed, possibly indicating the difficulties of standardizing the administration of orally used cannabis products.
One way cannabinoids may act to dampen the intensity of nociceptive signals in prolonged pain models is through their potentiating actions on descending inhibitory systems, which at least partly depends on the release of endogenous opioids. Descending inhibitory systems originate in the brainstem and are dynamically triggered following prolonged noxious insult [Millan 2002]. A double-blind, placebo-controlled, crossover study explored the analgesic and antihyperalgesic properties of the synthetic cannabinoid nabilone on long-lasting experimental heat pain, as well as its effects on descending pain inhibitory systems IRedmond 2008]. Single doses of 0.5 and 1 mg nabilone were administered to 10 men and 10 women. Primary outcome measures included average heat pain, temporal summation of heat pain, and drug-induced changes in the strength of descending analgesia. Administration of low-dose Nabilone did not act as an analgesic agent. However, a significant antihyperalgesic effect was observed in women only. No important AEs were observed during testing, and the most commonly observed side effects were dry mouth, red eyes, mild sedation, and euphoria.
Multiple sclerosis and spasticity
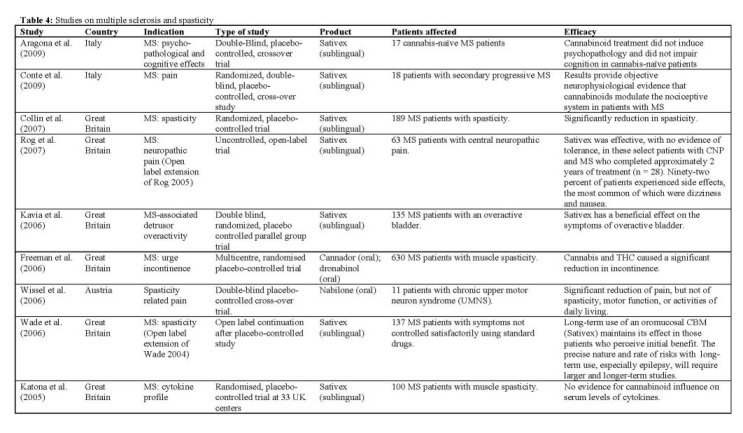
Although cannabinoids have been used mainly to alleviate symptoms of multiple sclerosis, there is also experimental evidence to suggest that they may be immunomodulatory. Cannabinoids are believed to be antiinflammatory, mainly through activation of the CB2 receptor, which is principally located peripherally, especially on leucocytes. CB2 activation may be associated with a Thl to Th2 shift. Consequently, there is some evidence that cannabinoids may be therapeutically useful in treating multiple sclerosis, which is generally believed to be an autoimmune condition. A clinical study IKatona 2005] investigated the nature of potential cannabinoid immunomodulation on serum samples obtained from patients with MS taking part in the CAMS study [Zajicek 2003, 2005]. Cannador and THC were used as study medication. With 657 patients recruited, this is to date the largest clinical trial per-
formed with any cannabis-based medicine. Serum samples of 100 subjects were available for analysis. Results did not demonstrate any significant effects of cannabinoids on the cytokine profiles examined, which included interferon-gamma (IFN-y), interleukin (IL)10, IL-12 and C-reactive protein. However, the standard deviations were large, so that relatively small but possibly clinically useful effects cannot be excluded from these results.
In 2004, Wade et aL performed a 10-week placebocontrolled study with 160 MS patients, administering Sativex using a self-titration dosing regimen. The study suggested that Sativex is an effective treatment for spasticity associated with MS, but the supporting data was not very strong. Therefore, the investigation was continued as an open label trial to monitor the safety and efficacy of long-term use of Sativex. A total of 137 MS patients who perceived to benefit from treatment entered the extension trial [Wade 2006]. Patients were assessed every eight weeks and were followed for an average of 434 days. This study concluded that patients with MS who derive symptom relief from Sativex in the first 10 weeks, generally maintain that relief over an extended period of treatment without any increase in dose. Patients tended to stabilize at a dose of approximately 11 sprays daily (equivalent to 30 mg THC and 28 mg CBD). Unwanted effects were common but rarely troublesome, and the majority was found to be unrelated to the treatment. Four patients experienced seizures, but all four were also taking other potentially epileptogenic drugs. Nevertheless, the relationship between Sativex (or other cannabis based medicines) and seizures warrants further investigation. Although only 67% of the initial number of subjects could be followed for at least one year on the medication, the obtained data nevertheless provides a large body of safety and tolerability data. A number of subjects who had received Sativex for at least one year were asked to participate in a planned abrupt interruption of the study medication for up to 14 days, in order to explore the possibility of a withdrawal syndrome and to determine whether MS-related symptoms would reappear. Of 25 patients participating, five resumed Sativex before the end of 14 days because of reemergence of marked MS symptoms. There was no consistent withdrawal syndrome on abrupt cessation, although just under half the patients experienced new symptoms that may have been related to withdrawal.
A study by Rog et aL 12005] compared the efficacy, safety, and tolerability of Sativex with placebo in relieving central neuropathic pain in 64 patients with MS. Patients could gradually self-titrate and the median dose used by subjects was equal to 25 mg of THC. The study concluded that Sativex is effective in reducing pain and sleep disturbance in the population studied. Patients in this study were taking, on average, two other medications, with limited efficacy given their baseline pain scores. Therefore, as adjunctive analgesic treatment, Sativex had a significant treatment effect. The numbers needed to treat (NINT) to achieve a 50% reduction in central pain in at least one patient was 3.7, similar to the value of 3.5 obtained in a previous thonabinol trial [Svendsen 2004]. The same group FRog 2007] continued their study with a long-term extension, treating MS patients for neuropathic pain with Sativex in an uncontrolled, open-label trial. Patients remained on a self-titration scheme, while maintaining their existing analgesia as required. Of 64 patients completing the original trial, 28 patients completed the extension with a mean duration of treatment of 839 days. In this group a relatively small but sustained reduction in pain was observed. Seventeen patients withdrew due to AEs; the most common of which were nausea, dizziness, weakness, and fatigue. Only two serious AEs were judged to be treatment-related. The mean dose of Sativex, and number of patients experiencing intoxication remained stable throughout the follow-up trial.
Lower urinary tract symptoms (LUTS) are very common symptoms of MS and are mainly due to neurogenic detrusor overactivity [Goldstein 1982], and often lead to bladder dysfunction. Anecdotal reports from MS patients have suggested that cannabis might have a beneficial effect on LUTS [Brady 2002]. Therefore, the effect of Cannador and pure THC on urge incontinence in patients with multiple sclerosis was determined in a multicentre, randomised placebo-controlled trial [Freeman 2006]. The data for this substudy was collected from the patient population of the CAMS study [Zajicek 2003], by asking subjects to complete incontinence diaries. Finally, 255 patients could be fully evaluated. Both Cannador and THC treatments showed significant effects over placebo in urge incontinence episodes. The authors hypothesized that cannabinoids relax the detrusor smooth muscle during filling, thereby improving neurogenic detrusor overactivity. Further support for a positive treatment effect comes from the measurement of lower volumes of involuntary urine loss in the active treatment groups. Because this was an "add-on" study to the CAMS study, which was assessing spasticity, patients were selected on this symptom rather than on incontinence. A proper trial set up specifically to test for incontinence may therefore yield more robust results. Nevertheless, it has been shown that even a modest 25% reduction in urge incontinence might be clinically significant [Coyne 2005].
Another, smaller, study was performed to determine the effects of Sativex treatment on the overactive bladder in MS IKavia 2006]. Patients were treated over a period of 8 weeks, in order to detect an improvement in urgency incontinence. Although the study failed to show a reduction in daily incontinence at the end of the study, Sativex was superior to placebo for nocturia. This effect was greater for more severe disease, and a substantial number of patients became nocturia free on the active treatment. Patients on Sativex were three times more likely to report an improvement of >30% compared to placebo. Active treatment was well tolerated, and the most common adverse effects were dizziness, urinary tract infection, and headache.
Because THC was reported to add benefit in the treatment of pain in patients with MS, the question arose whether synthetic cannabinoids with lower potential for psychotropic side effects could be effective as well. A double-blind, placebo-controlled, cross-over trial was performed to evaluate the safety and efficacy of low dose treatment with nabilone (1 mg per day) on spasticity-related pain IWis sel 2006]. Patients all suffered from chronic upper motor neuron syndrome (UMNS) not sufficiently correctable by conventional treatment. Results showed a significant decrease of pain under nabilone after 4 weeks of treatment, while spasticity, motor function and activities of daily living did not change. Although one patient dropped out because of weakness of lower limbs which could be attributed to nabilone, the other side effects observed in the present study were stated as mild and easily tolerable, or not related to the treatment. The study also assessed neuropsychological parameters relevant for driving ability in a subset of patients [Kurtzhaler 2005], but no cognitive side effects were found in domains of attentional performance, psychomotor speed, and mental flexibility.
In a randomized, placebo-controlled trial on the efficacy and tolerability of Sativex, 189 subjects with definite MS and spasticity were treated over a 6 week period. Subjects were allowed to self-titrate their daily dose, which resulted in a mean dose of ca. 25 mg of THC and of CBD (9.4 sprays) per day. Results rated Sativex significantly more effective than placebo in relieving spasticity ICollin 2007]. Of the Intention to Treat (ITT) population, 40% of the subjects achieved >30% improvement from baseline. The secondary outcomes did not achieve statistical significance but were all in favour of Sativex. The low rate of subject withdrawal due to AEs in this study may seem surprising given that the dose of THC, present in the cannabis extract, was being taken in mean daily doses in excess of 25 mg, considerably more than was given in most other published studies. However, this may reflect the presence of CBD, which is known to modify some of the psychoactive effects of THC, so that THC as part of a cannabis extract may become better tolerated than THC as a single molecule [Zuardi 1982].
In a group of 18 patients with secondary progressive MS, a study was performed to identify the neurotransmitter system involved in the pain control by cannabinoids in MS [Conte 2009]. The flexion reflex method was used, an objective tool for assessing pain threshold, pain pathways and the neurotransmitter system involved in pain control [Santhini 1993]. After administration of Sativex, at a mean dose of 8 sprays daily (ca. 20 mg THC and CBD), a significant effect was observed on the parameters recorded. Also the patients' VAS pain scores decreased, although not significantly. It was concluded that cannabinoids modulate human pain perception mainly by acting at the pre-motorneuronal level in the spinal cord. Cannabinoids, like opioids, could act by decreasing neurotransmitter release.
Although no significant cognitive deficits were reported in frequent but moderate users of cannabis [Jager 2006] the persistent effects of cannabis on cognition remain uncertain [Verdejo-Garcia 2004]. Therefore, the primary aim of a double-blind, placebo controlled, crossover study performed by Aragona et aL 12009] was to explore the onset of psychopathological symptoms and cognitive deficits in cannabis-naïve patients with MS treated with Sativex for relieving their spasticity. The mean daily dose used by self-titration corresponded to ca. 22 mg of THC. The effects on psychopathology were evaluated after 3 weeks of treatment. During the study, plasma levels of THC and CBD were monitored. Cannabinoid treatment did not induce psychopathology and did not impair cognition in subjects. Also the effects of cannabinoids on quality of life, fatigue, and motor function of MS patients were non-significant; however, the positive correlation between plasma levels of THC and psychopathological scores suggests that at dosages higher than those used in therapeutic settings, interpersonal sensitivity, aggressiveness, and paranoiac features might arise. All subjects finished the study. Safety and tolerability were generally good, drug tolerance and dose increasing were not reported during the trial, and desire for Sativex or abuse was not present at follow-up.
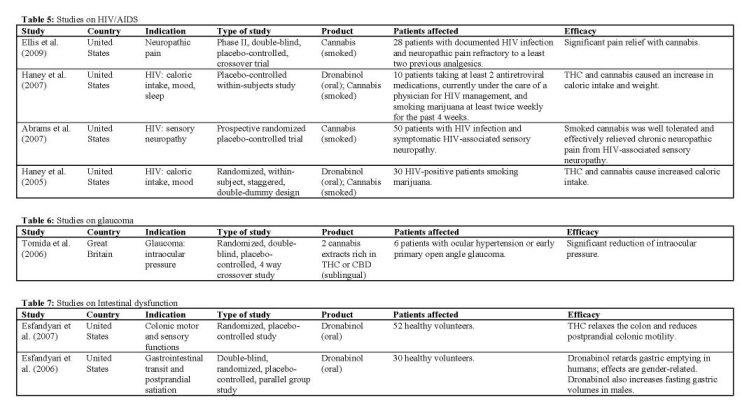
HIV/AIDS
In two studies, Haney et al. demonstrated that smoked cannabis, and oral dronabinol, stimulates appetite in already experienced cannabis smokers. In the first study Illaney 2005], using only acute doses, it was found that for experienced cannabis smokers with clinically significant wasting, both dronabinol (at acute doses at least four to eight times the current recommendation) and cannabis produced substantial and comparable increases in food intake without causing major adverse effects. Caloric intake was only increased in the group with significant wasting, but not in a control group of HIV patients without signs of wasting. Only the highest dose of dronabinol (30 mg) was poorly tolerated, producing at least one adverse effect (e.g., headache, nausea, overintoxication) in 20% of the participants, suggesting that this (oral) dose may be too high, even among regular cannabis smokers.
The second study Illaney 2007] showed that also repeated long-term doses of both dronabinol (up to 10 mg daily) and smoked cannabis (up to 3.9% THC) were well tolerated and produced substantial and comparable increases in food intake. Both drugs dosedependently increased daily caloric intake and body weight, without causing disruptions in psychomotor functioning. For the high-dose dronabinol and cannabis conditions, this resulted in a significant increase in body weight within 4 days (>1 kg). Both active treatments increased daily food intake by increasing the number of times participants ate throughout the day, without altering the number of calories consumed during each eating occasion. Increased food intake paralleled increased ratings of intoxication (generally rated
as positive by patients) for all cannabinoid conditions, except for the low dose of dronabinol (5 mg).
HIV-associated sensory neuropathy is the most common peripheral nerve disorder complicating HIV-1 infection, most often defined by hyperalgesia and allodynia. Abrams et aL 12007] determined the effect of smoked cannabis on this condition. Patients were randomly assigned to smoke either cannabis or identical placebo cigarettes three times daily for 5 days. It was found that smoked cannabis reduced daily pain significantly compared to placebo; the number needed to treat (NINT) in order to achieve a >30% pain reduction (commonly seen as a clinically relevant improvement) among all completing patients was 3.6. These findings are comparable to oral drugs routinely used for chronic neuropathic pain, such as Gabapentin [Backonja 1998]. Cannabis also reduced some types of experimentally induced hyperalgesia in the same patients. Although the active treatment was well tolerated, side effects ratings were higher in patients in the cannabis group for anxiety, sedation, disorientation, confusion, and dizziness. No serious AEs were reported, and no patient withdrew from the study because of AEs.
Despite management with opioids and other pain modifying therapies, neuropathic pain continues to reduce the quality of life and daily functioning in HIV-infected individuals. In a randomized cross-over trial, smoked cannabis at maximum tolerable dose (1-8% THC), significantly reduced neuropathic pain intensity in HIV-associated distal sensory predominant polyneuropathy (DSPN) compared to placebo when added to stable concomitant analgesics FEujs 2009], Among the completers, pain relief was greater with cannabis than placebo. Using verbal descriptors of pain magnitude from the Descriptor Differential Scale (DDS), cannabis was associated with an average reduction of pain intensity from 'strong' to 'mild to moderate'. Also, cannabis was associated with a sizeable (46%) and compared to placebo (18%) significantly greater proportion of patients who achieved a >30% reduction in pain. Smoked cannabis was generally well tolerated and effective when added to concomitant analgesic therapy in these patients. The frequency of some non-treatment-limiting side effects was greater for cannabis than placebo. These included concentration difficulties, fatigue, sleepiness or sedation, increased duration of sleep, reduced salivation, and thirst. Although most side effects were mild and self-limited, two subjects experienced treatment-limiting toxicities.
Glaucoma
There is increasing evidence suggesting that cannabinoids may lower TOP primarily by influencing aqueous humor production and outflow, through activation of the C131 receptor. In glaucoma, the final pathway leading to visual loss is the selective death of retinal ganglion cells through apoptosis. Recent studies have documented the neuroprotective properties of cannabinoids independently of their effect on TOP [listed in Tomida 2006]. But despite these promising results, in recent years only a single clinical trial has been added to the scientific literature.
Tomida et aL 12006] performed a pilot study to assess the effect on TOP, and the safety and tolerability of a low dose of THC and CBD. Although topical administration (eye drops) of cannabinoids would be ideal for glaucoma, this type of application has been associated with irritation and corneal damage [Jay 1983]. Therefore, an oromucosal spray was used because it has been shown to have a satisfactory pharmacokinetic profile and has been well tolerated in clinical studies [Guy 2003]. Patients with ocular hypertension or early primary open angle glaucoma received single dose standardized cannabis extracts, containing either 5 mg THC, 20 mg CBD, 40 mg CBD, or placebo. Two hours after administration of THC, the TOP was significantly lower than after placebo, returning to baseline level after 4 hours. CBD administration did not reduce the TOP at any time with either of the two doses studied. Instead, the higher dose of CBD (40 mg) produced a transient elevation of TOP at 4 hours after administration. One patient experienced mild psychotropic side effects, but there were no serious AEs.
Intestinal dysfunction
Two controlled clinical trials have been performed in the period covered by this review. The first study lEsfandyari 2006] evaluated the effects of dronabinol on gastrointestinal transit, gastric volume and satiation in healthy volunteers, who were randomly assigned to receive three doses of THC (5 mg) or placebo over a period of 24h. The results suggested that THC administration was associated with a significant delay in gastric emptying of a standard solid and liquid meal, and there was a suggestion of a gender effect: THC significantly slowed gastric emptying in females, but not in males, which is consistent with earlier findings [Bateman 1983]. In contrast, THC increased fasting gastric volumes specifically in males. The data obtained suggested that the antiemetic effect of cannabinoids may not be due to a direct effect on gastric accommodation or sensation, but rather to a central modulation of perception.
A second study by the same group lEsfandyari 2007]
aimed to compare the acute effects of single dose thonabinol (7.5 mg) versus placebo on colonic sensory and motor functions in healthy adults. The study demonstrated that THC was associated with relaxation of the colon and inhibition of the increase in tone after the meal. It was concluded that the potential for CB agonists to modulate colonic motor function in diarrheal disease such as irritable bowel syndrome deserves further study. As in the previous trial [Esfandyari 2006], the study observed greater effect of THC on gastric emptying prolongation in female volunteers than in males. The significance of the observed genderrelated differences is unclear.
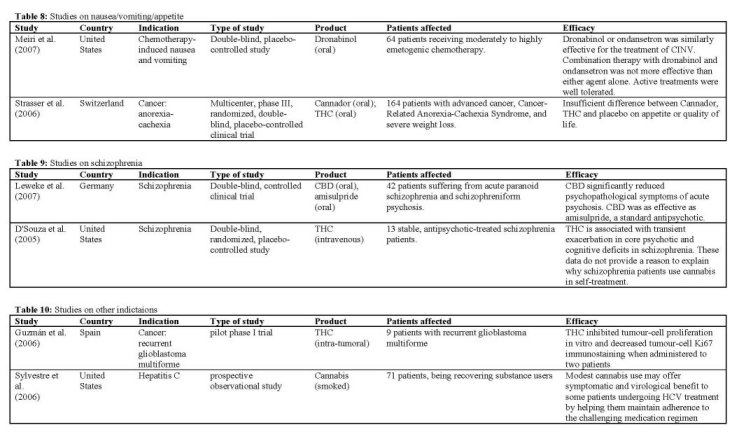
Nausea-vomiting-appetite
The purpose of the placebo-controlled study by
Strasser et aL 12006] was to compare the effects of Cannador and THC on appetite and quality of life in patients with cancer-related anorexia-cachexia syndrome (CACS). Adult patients with significant weight loss were treated with Cannador (standardized for 2.5 mg THC and 1 mg CBD) or THC (2.5 mg) twice daily for 6 weeks. Appetite, mood, and nausea were monitored daily. Cannador at the oral dose administered was well tolerated by the study subjects. Results showed no significant differences between the three arms for appetite, quality of life, or cannabinoid-related toxicity. Increased appetite was reported by 73%, 58%, and 69% of patients receiving Cannador, THC, or placebo, respectively. Finally, an independent data review board recommended termination of recruitment because of insufficient differences between study arms. A large number of adverse effects were observed, but there were no differences between treatment arms, and only a minority of adverse effects was found to be linked to study medication. Authors assumed that the study medications were underdosed.
Delayed chemotherapy-induced nausea and vomiting (C1NV), defined as nausea and vomiting occurring more than 24 hours after chemotherapy and lasting for up to 1 week, is common, with at least 50% of patients experiencing it following moderately emetogenic chemotherapy. The impaired quality of life imparted by C1NV can affect treatment outcomes when patients refuse chemotherapy because of severe AEs. A recent study IMeiri 2007] evaluated the efficacy of thonabinol versus ondansetron in delayed C1NV. Over the course of 2-5 days after receiving chemotherapy, subjects received an increasing dose of up to 20 mg thonabinol daily, either alone, or in combination with ondansetron. Efficacy of thonabinol alone was comparable with ondansetron, and combination therapy did not provide benefit beyond that observed with either agent alone. Nevertheless, specifically on day 1 after chemotherapy, significantly greater efficacy on intensity of nausea was demonstrated in the combined active treatment group versus placebo. Active treatments were well tolerated. The highest rate of CNS-related AEs (dizziness and fatigue) was found in patients receiving combination therapy, while the incidence of these events in the THC group was low. Also, it was found that quality of life was most improved in patients receiving thonabinol compared with patients in the other treatment groups.
Schizophrenia
An explorative, 4-week, double-blind, controlled clinical trial was performed by Leweke 12007] on the anti-psychotic properties of CBD in acute schizophrenia compared to the standard antipsychotic amisulpride. Furthermore, side-effects and anxiolytic capabilities of both treatments were investigated. Forty-two patients fulfilling DSM-W criteria of acute paranoid schizophrenia or schizophreniform psychosis participated in the study. Both treatments were associated with a significant decrease of psychotic symptoms after 2 and 4 weeks. However, there was no statistical difference between both treatment groups. In contrast, cannabidiol induced significantly less side effects (EPS, increase in prolactin, weight gain) when compared to amisulpride. It was concluded that CBD proved substantial antipsychotic properties in acute schizophrenia.
In another clinical study ID'Souza 2005], the behavioral, cognitive, motor, and endocrine effects of up to 5 mg intravenous THC were characterized in stable, antipsychotic-treated schizophrenia patients. These data were compared with effects in healthy subjects reported elsewhere. It was found that THC transiently exacerbated a range of positive and negative symptoms, perceptual alterations, cognitive deficits, and medication side effects associated with schizophrenia without producing any obvious "beneficial" effects. The data do not provide a reason to explain why schizophrenia patients use or misuse cannabis. Furthermore, schizophrenia patients were more vulnerable to THC effects on learning and memory than healthy subjects. The enhanced sensitivity to the cognitive effects of THC warrants further study into whether brain cannabinoid receptor dysfunction contributes to the pathophysiology of the cognitive deficits associated with schizophrenia.
Other indications
The effects of intratumoral THC IGuzmán 2006] were studied on 9 patients with recurrent glioblastoma multiforme. A dose escalation regimen for THC administration was assessed. Cannabinoid delivery was safe and could be achieved without overt psychoactive effects. The treatment was found to inhibit tumour-cell proliferation in vitro and to decrease tumour-cell Ki67 immunostaining in two patients. The fair safety profile of THC, together with its possible antiproliferative action on tumour cells reported here and in other studies, may set the basis for future trials aimed at evaluating the potential antitumoral activity of cannabinoids.
[Sylvestre 2006] performed a study on 71 patients suffering from hepatitis C, all being recovering heroin users consuming cannabis on their own account. It was found that modest use of smoked cannabis may offer symptomatic and virological benefit to some patients undergoing viral treatment by helping them maintain adherence to the challenging medication regimen. The lack of dose response in this study argues against specific receptor- or metabolism-related effects, and suggests instead that cannabis exerted its benefit by nonspecific improvements in symptom management. It must be noted that the authors point out a number of limitations that warrant caution in the interpretation of this study.
Discussion
This review is intended to support the discussion on the question whether there is currently enough clinical data to accept cannabis and cannabinoids as drugs in certain indications. In the review by Ben Amar [2006], a
therapeutic potential of cannabinoids was concluded for a range of disorders. Based on the data presented here, covering the period 2005-2009, it is possible to confirm that cannabinoids exhibit a strong therapeutic potential mainly as analgesics in chronic neuropathic pain, appetite stimulants in debilitating diseases (cancer and AIDS), as well as in the treatment of multiple sclerosis. For each of the 8 main indications discussed in this review, the general conclusions are discussed below.
It may be interesting to note that in the last few years, some well-designed studies on the effects of smoked cannabis have been released, mainly on HIV/AIDS. This is of specific interest because most patients administer their medicinal cannabis by smoking. The studies particularly show a benefit on neuropathic pain and appetite. Obviously, the noxious pyrolytic byproducts released through combustion remain a public health deterrent to the use of smoked cannabis. However, specific herbal vaporizers have been devised to provide a safer and more efficient delivery system for inhaling cannabis. It is reasonable to assume that future clinical trials will utilize this alternative delivery method.
Pain
Although cannabinoid-induced analgesia is now wellrecognized in animal models, evidence of its analgesic properties in humans is less conclusive. Interestingly, trials involving pain patients with neuropathic-like features (e.g. multiple sclerosis, neuropathic pain and fibromyalgia) have produced mostly positive results, whereas studies measuring the efficacy of cannabinoids for acute pain (e.g. postoperative pain) have generated mostly negative results. For that reason, experimental pain and chronic (neuropathic) pain are discussed in separate sections. It has been demonstrated that endocannabinoids produced in the spinal cord can enhance pain by dampening the synapses of inhibitory interneurons that usually prevent the perception of innocuous stimuli as painful [Christie and Mallet 2009]. The painpromoting action of endocannabinoids wanes during the development of chronic pain that is induced by inflammation or nerve injury. This can explain the differences observed in clinical studies with cannabinoids on acute and chronic pain.
The results of the clinical trials on chronic and neuropathic pain conditions are equivocal. A wide range of cannabis-based medicines exhibit analgesic effects on different forms of pain. THC, nabilone, Sativex, Cannador and even smoked cannabis have been used in these studies, either alone or in addition to existing analgesia. The large majority of adverse effects were mild or moderate. Chronic neuropathic pain is a common and difficult to treat condition that has limited treatment options. As a consequence, even modest clinical effects may be relevant. Studies with cannabinoids should therefore be regarded as highly significant for the intended patient population. Clearly, the optimal type of cannabinoids and administration route may differ for each indication.
Acute types of pain did not respond as well to cannabinoids. For postoperative pain management, the use of THC or nabilone did not reveal a positive effect on pain scores and a higher dose of nabilone (2 mg) actually increased pain scores. The use of Cannador, a standardized extract containing both THC and CBD, was more successful, and dose-dependently decreased postoperative pain. The presence of CBD may modulate the effects of THC (e.g. by changing the pharmacokinetic profile of THC and its metabolites), and it may also be possible that CBD has an effect on pain by itself as shown in an animal model of neuropathic pain [Costa et al. 2007].
A crucial caveat in the study of cannabis or cannabinoids in experimental pain models is that the data is mainly collected with healthy, regular marijuana users who smoke acute doses in a controlled laboratory situation and are exposed to artificial pain stimuli. Obviously, it is not possible to predict whether chronically ill patients taking cannabinoids for pain relief would respond similarly. The respective mechanisms underlying the whole variety of chronic pain syndromes may considerably differ from acute nociception. It has previously been reported that in rats, cannabinoid CB 1 receptors are upregulated in chronic neuropathic pain and therefore could lead to an increased analgesic effect of THC in chronic pain [Siegling 2001]. It is interesting to note that a selective effect on women was observed in some pain studies. This may be an indication that certain cannabinoids may help alleviate chronic pain conditions which predominantly affect women, such as fibromyalgia.
Experimental pain studies often show that THC-induced analgesia is accompanied (and outlasted) by side-effects such as sedation. At doses producing substantial biological exposure, the antinociceptive effects of cannabis - although statistically significant - are often rather weak compared with motor-impairing and subjective effects. Nevertheless, in certain groups of chronically ill patients with severe enough symptoms, and without further options for treatment, even this weak effect on pain may be significant enough.
In previous animal and human studies, it has been shown that cannabinoids and opioids have synergistic actions on pain control [Iversen 2003; Lynch and Clark 2003; Maldonado and Valverde 2003], but for chronic pain this could not be firmly confirmed in the clinical trials reported here. More study is needed to evaluate the combined analgesic effects of both types of drugs.
Multiple sclerosis and spasticity
In clinical trials, more patients have been treated with cannabinoids for MS then for any other indication. Symptomatic therapy for MS often provides inadequate relief and can be limited by toxicity. As a consequence, people with multiple sclerosis have experimented with many alternative therapies, including cannabis, to ease their physical problems. There is much anecdotal suggestion that cannabis and cannabinoids, have beneficial
effects on disease-related pain, bladder symptoms, tremor, and particularly spasticity, but until recently, little scientific evidence existed for their efficacy. In the period covered by this review, nine studies have been released on the effect of cannabinoids on MS symptoms. Most studies were done with Sativex, which is currently approved only in Canada, and the largest studies have been conducted with Cannador and thonabinol.
MS is one of the few conditions where long-term extension studies have been performed with cannabisbased medicines. When assessing clinical results, it should be acknowledged that the degree of evidence for many of the commonly used drugs to combat MS symptoms is weak. A Cochrane review [Shakespeare 2003] of antispasticity agents for multiple sclerosis concluded that the paucity of evidence meant no recommendations could be made to guide prescribing, and that better outcome measures need to be developed. It may therefore not be surprising that it has proven hard to collect evidence for the efficacy of cannabis in the treatment of MS.
The current studies presented in this review provide us
with cautious optimism that Sativex, but also Cannador, THC and nabilone, can improve the symptoms of spasticity in MS sufferers, specifically for the treatment of spasticity, pain and incontinence. Often the improvements were gained over and above the concomitant anti-spasticity medication being taken by the subjects during the study. In those patients perceiving initial benefit from their medication, the positive effects often persisted in longer term extension trials without tolerance. This is representative of clinical practice, where only patients who consider a treatment beneficial will continue taking it. Cannador or THC did not show any detectable effects on a range of cytokines that influence inflammation in serum samples of MS patients.
HIV/AIDS
The primary constituent of cannabis, THC, is approved by the Food and Drug Administration (FDA) for oral administration as appetite stimulant in the case of anorexia associated with weight loss in patients with HIV/AIDS. Studies on the effects of cannabinoids in patients with HIV are particularly important given that they constitute one of the largest groups using thonabinol and cannabis for medicinal reasons [Institute of Medicine 1999], and a considerable proportion of those with HIV currently smoke cannabis. Reasons for smoking cannabis cited by patients include countering the nausea, anorexia, stomach upset, and anxiety associated with the disease and with antiretroviral therapy.
The four studies presented here all used smoked cannabis, but also THC, and clearly showed the beneficial effects on pain, appetite and weight gain. Although cannabinoids tend to increase fat rather than the more wanted lean muscle mass [Abrams 2003], HIV patients who are able to maintain stable weight often report improved quality of life [Beal 1995]. Overdosing effects were relatively common, because the exact dose of cannabinoids is relatively difficult to control in smoked studies, compared to oral administration.
Glaucoma
Glaucoma is one of the leading causes of blindness in the world, affecting about 70 million people worldwide. As glaucoma is a chronic disease lacking a cure, the quest for new ocular hypotensive agents is important for its treatment, and these agents are likely to remain frontline therapy for the foreseeable future. Since the early 1970s, it was reported that smoking cannabis cigarettes could lower intraocular pressure (TOP) by up to 45% [Hepler & Frank 1971]; later works showed that THC lowered TOP when given intravenously, orally or by inhalation [Ben Amar 2006]. Since these early observations, numerous studies have been conducted confirming that different cannabinoids, including THC, CBD, cannabigerol, endogenous cannabinoids, and some synthetic cannabinoids, can reduce TOP when administered systemically and topically [listed in Tomida 2006]. In addition to the reduction of TOP THC may increase blood circulation in the retina, which was demonstrated in an open study [Plange et al. 2007], and is known to be neuroprotective, which both may increase survival of the optical nerve. Only one single controlled clinical study was added to the literature in the past years. The modest reduction of TOP observed after oromucosal administration of THC was not deemed to be clinically relevant. An important goal of further research may be to determine the additive effects of cannabinoids with the anti-glaucoma agents available.
Intestinal dysfunction
Cannabinoid receptor (CB) stimulation inhibits colon motility and increases food intake in rodents. However, effects of CB stimulation in human gastrointestinal (GI) tract are largely unclear. In vitro studies have suggested that cannabinoids delay transit in human colon and ileum [Manara 2002]. In general, reports of effects of cannabinoids on GI transit and sensation in humans in vivo are sparse, and the role of stomach function in the appetite-stimulating and anti-emetic effects of cannabinoid agonists is unclear. The two studies discussed here indicate that THC administration was associated with a significant delay in gastric emptying, relaxation of the colon and inhibition of the increase in tone after the meal. The obtained data may help to better understand the effects of cannabinoids in nausea, vomiting and appetite. In both studies, a greater effect of THC was observed on gastric emptying prolongation in female volunteers than in males. The significance of the observed gender-related differences is yet unclear.
Nausea, vomiting and appetite
Besides the use as an appetite stimulant for AIDS patients, THC is FDA approved in the USA as an antiemetic for cancer patients undergoing chemotherapy.
One study showed no significant effect of either Cannador (containing THC and CBD) or THC on appetite and nausea in cancer patients, but study medications were obviously underdosed since there was no difference of side-effects compared to placebo. A second study demonstrated an effect in delayed chemotherapyinduced nausea and vomiting (C1NV), and this effect was comparable to the standard drug ondansetron. The data suggest that the addition of THC directly before and after chemotherapy may offer more benefit than the standard regimen alone taken before chemotherapy.
Schizophrenia
The human endocannabinoid system interacts with various neurotransmitter systems and the endocannabinoid anandamide was found significantly elevated in CSF and inversely correlated topsychopathology in patients with schizophrenia [Giuffrida 2004] providing a link to the neurobiology of the disease. The major herbal cannabinoid compound CBD was suggested recently to be a re-uptake inhibitor of anandamide. In a study using purified CBD, it was found that this nonpsychoactive compound shows substantial antipsychotic properties in acute schizophrenia, with an efficacy comparable to amisulpride. This is in line with the suggestion of an adaptive role of the endocannabinoid system in paranoid schizophrenia, and raises further evidence that endocannabinoid system may represent a valuable target for antipsychotic treatment strategies. Another study using high doses of intravenous THC caused schizophrenia-like symptoms.
Other indications
Most of the experiments performed so far in animal models of cancer have evidenced a tumour growthinhibiting action of cannabinoids (Guzmán, 2003). The study by Guzmán et aL described in this review was the first clinical study aimed at evaluating cannabinoid antitumoral action. Owing to obvious ethical and legal reasons, this pilot study was conducted in a cohort of terminal patients harbouring actively growing recurrent tumours. In view of the fair safety profile of THC, together with its possible antiproliferative action on tumour cells reported here and in other studies (Guzman, 2003), it would be desirable that additional trials - on various types of tumours - were run to determine whether cannabinoids - as single drugs or in combination with established antitumoral drugs - could be used, other than for their palliative effects, to inhibit tumour growth.
Another indication that was clinically studied for the first time in recent years was hepatitis C. Although hepatitis C vins (HCV) treatment outcomes have improved dramatically over the past decade, the intolerability of interferonlribavirin combination therapy remains a barrier to treatment success. Faced with severe treatment-related side-effects that respond inadequately to conventional medications, some patients turn to cannabis for symptom relief. Although widespread restrictions limit the ease with which medicinal cannabis use can be formally studied, the pervasive use of cannabis by patients during HCV treatment provided a means for an observational study of its potential risks and benefits. Despite its shortcomings, the study by Sylvestre et aL [2006] begins to answer some of the key questions that arise about the use of cannabis during HCV treatment. The results of this observational study suggest that at least moderate use of cannabis during HCV treatment can improve adherence by increasing the duration of time that patients remain on therapy. However, because the benefits of heavy cannabis use were less apparent, the authors could not rule out the possibility that detrimental biological or immunological mechanisms may be relevant at higher levels of consumption.
A series of studies have previously [Ben Amar 2006] shown promising effects of THC on tics associated with Tourelles syndrome as well as its associated behavioral problems such as obsessive-compulsive behavior, providing a reason for careful optimism in the treatment of this poorly understood condition. However, no new data has been published in recent years. Also no new clinical studies were released in recent years on the use of cannabinoids for epilepsy.
References
1 Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, Deeks SG, Mitchell TF, Mulligan K, Bacchetti P, McCune JM, Schambelan M. Short-term effects of cannabinoids in patients with HIV- 1 infection: a randomized, placebo-controlled clinical trial. Ann. Intern. Med. 2003;139(4):258-266.
2. Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, Petersen KL. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology 2007;68(7):515-521.
3. Agurell S, Carlsson S, Lindgren JE, Ohlsson A, Gillespie H, Hollister L. Interactions of delta 1tetrahydrocannabinol with cannabinol and cannabidiol following oral administration in man. Assay of cannabinol and cannabidiol by mass fragmentography. Experientia 1981;37(10):10901092.
4. Aragona M, Onesti E, Tomassini V, Conte A, Gupta S, Gilio F, Pantano P, Pozzilli C, Inghilleri M. Psychopathological and cognitive effects of therapeutic cannabinoids in multiple sclerosis: a double-blind, placebo controlled, crossover study. Clin. Neuropharmacol. 2009;32(l):41-47.
5. Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, LaMoreaux L, Garofalo E. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA 1998;280:1831-1836.
6. Bateman DN. Delta-9-tetrahydrocannabinol and gastric emptying. Br. J. Clin. Pharmacol. 1983; 15:749-751.
7. Beal J, Flynn N. AIDS-associated anorexia. J.
Physicians Assoc. AIDS Care 1995;2(1):19-22.
8. Beaulieu P. Effects of nabilone, a synthetic cannabinoid, on postoperative pain. Can. J. Anaesth. 2006;53(8):769-775.
9. Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J. Ethnopharm. 2006;105:1-25.
10. Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde SDE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001;134(4):845-52.
11. Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford) 2006;45(1):50-52.
12. Bornheim LM, and Grillo MP. Characterization of cytochrome P450 3A inactivation by cannabidiol: possible involvement of cannabidiolhydroxyquinone as a P450 inactivator. Chem. Res. Toxicol. 1998;11(10):1209-1216.
13. Bonheim LM, Kim KY, Li J, Perotti BY, Benet LZ. Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain. Drug Metab. Dispos. 1995;23(8): 825-831.
14. Brady CM, DasGupta R, Wiseman OJ, Dalton CM, Berkley KJ, Fowler CJ. The effect of cannabis based medicinal extract on lower urinary tract dysfunction in advanced multiple sclerosis: preliminary results. Journal of Neurology, Neurosurgery and Psychiatry 2002;72:139
15. Christie MJ, Mallet C. Endocannabinoids can
open the pain gate. Sci. Signal. 2009;2(88):pe57.
16. Collin C, Davies P, Mutiboko 1K, Ratcliffe S; Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur. J. Neurol. 2007;14(3):290-296.
17. Conte, A, Marini Bettolo C, Onesti E, Frasca V, lacovelli E, Gilio F, Giacomelli E, Gabriele M, Aragona M, Tomassini V, Pantano P, Pozzilli C, Jnghilleri M. Cannabinoid-induced effects on the nociceptive system: A neurophysiological study in patients with secondary progressive multiple sclerosis. European Journal of Pain 2009;13:472477.
18. Corchero J, Manzanares J, Fuentes JA. Cannabinoidlopioid crosstalk in the central nervous system. Crit. Rev. Neurobiol. 2004;16:159-172.
19. Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 2007;556(13):75-83.
20 Coyne KS, Matza LS, Thompson CL. The responsiveness of the Overactive Bladder Questionnaire (OAB-q). Quai. Life Res. 2005;14:849855.
21 Craft RM. Sex differences in behavioral effects of cannabinoids. Life Sci. 2005;77:2471-2478.
22 DSouza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, Gueorguieva R, Cooper TB, Krystal JH. Delta-9tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol. Psychiatry 2005;57(6):594-608.
23 Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, Bentley H, Atkinson JH. Smoked Medicinal Cannabis for Neuropathic Pain in HIV: A Randomized, Crossover Clinical Trial. Neuropsychopharmacology 2009;34(3): 672-680.
24 Esfandyari T, Camilleri M, Busciglio I, Burton D, Baxter K, Zinsmeister AR. Effects of a cannabinoid receptor agonist on colonic motor and sensory functions in humans: a randomized, placebocontrolled study. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293(1):G137-G145.
25 Esfandyari T, Camilleri M, Ferber J, Burton D, Baxter K, Zinsmeister AR. Effect of a cannabinoid agonist on gastrointestinal transit and postprandial satiation in healthy human subjects: a randomized, placebo-controlled study. Neurogastroenterol. Motu. 2006;18(9):831-838.
26 Farrar 1f, Young W, La Moreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2000;94:149158.
27 Frank B, Serpell MG, Hughes J, Matthews IN, Kapur D. Comparison of analgesic effects and patient tolerability of nabilone and dihydrocodeine for chronic neuropathic pain: randomised, crossover, double blind study. BMJ 2008;336(7637):199-201.
28 Freeman RM, Adekanmi O, Waterfield MR, Waterfield AE, Wright D, Zajicek J. The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicentre, randomised placebo-controlled trial (CAMS-LUTS). Int. Urogynecol. J. Pelvic Floor Dysftinct. 2006;17(6):636641.
29 Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, Klosterkötter J, Piomelli D. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology 2004;29(11):2108-2114.
30. Goldstein I, Siroky MB, Sax DS, Krane RJ. Neurourologic abnormalities in multiple-sclerosis. J. Urol. 1982; 128(3):541-545.
31. Guy GW, Robson P. A phase 1, double blind, three way crossover study to assess the pharmacokinetic profile of cannabis based medicinal extract (CBME) administered sublingually in variant cannabinoid ratios in normal healthy male volunteers. J. Cannabis Ther. 2003;3:121-152.
32. Guzmán M, Duarte MJ, Blázquez C, Ravina J, Rosa MC, Galve-Roperh I, Sanchez C, Velasco G, González-Feria L. A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br. J. Cancer 2006;95(2):197-203.
33. Guzmán M. Cannabinoids: potential anticancer
agents. Nat. Rev. Cancer 2003;3:745-755.
34. Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, Foltin RW. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J. Acquir. Immune Defic. Syndr. 2007;45(5):545-554.
35. Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology (Ben) 2005;181(1): 170-178.
36. Hepler RS, Frank JR. Marihuana smoking and
intraocular pressure. JAMA 1971;217(10):1392.
37. Holdcroft A, Maze M, Doré C, Tebbs S, Thompson S. A multicenter dose-escalation study of the analgesic and adverse effects of an oral cannabis extract (Cannador) for postoperative pain management. Anesthesiology 2006; 104(5):10401046.
38. Hunt CA, Jones RT, Herning RI, Bachman J. Evidence that cannabidiol does not significantly alter the pharmacokinetics of tetrahydrocannabinol in man. J. Pharmacokinet. Biopharm. 1981; 9(3):245-260.
39. Institute of Medicine, Marijuana and medicine: assessing the scientific base. National Academy Press, Washington DC, MD. 1999.
40. Iversen L. Cannabis and the brain. Brain
2003;126:1252-1270.
41. Jaeger W, Benet LZ, Bornheim LM. Inhibition of cyclosporine and tetrahydrocannabinol metabolism by cannabidiol in mouse and human microsomes. Xenobiotica 1996;26(3):275-284.
42. Jager G, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. Long-term effects of frequent cannabis use on working memory and attention: an fMRJ study. Psychopharmacol (Ben) 2006;185:358-368.
43. Jay WM, Green K. Multiple drop study of topically applied 1% delta-9-tetrahydnocannabinol in human eyes. Arch Ophthalmol. 1983;101:591593.
44. Katona S, Kaminski E, Sanders H, Zajicek J.Cannabinoid influence on cytokine profile in multiple sclerosis. Clin. Exp. Immunol. 2005; 140(3):580-585.
45 Kavia R, De Ridder D, Sarantis N, Constantinescu C, Fowler. Randomised controlled trial of cannabis based medicine (CBM, Stativex®) to treat detrusor overactivity in multiple sclerosis. Neurourology and Urodynamics 2006;25:166166.
46 Kraft B, Frickey NA, Kaufmann RM, Reif M, Frey R, Gustorff B, Kress HG. Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology 2008;109(1):101-1 10.
47 Leweke FM, Koethe D, Gerth CW, Nolden BM, Schreiber D, Hansel A, Neatby MA, Juelicher A, Hellmich M, Klosterkötter J. Cannabidiol as an antipsychotic. A double-blind, controlled clinical trial on cannabidiol vs. amisulpride in acute schizophrenia. Abstract for oral sessions / European Psychiatry 2007;22:S 14.02.
48 Lynch MA, Clark AJ. Cannabis reduces opioid dose in the treatment of chronic non-cancer pain. Journal of Pain and Symptom Management 2003;25:496-498.
49 Maldonado R, Valverde O. Participation of the opioid system in cannabinoid-induced anticiception and emotional-like responses. European Neuropsychopharmacology 2003;13:401-410.
50 Manara L, Croci T, Guagnini F, Rinaldi-Carmona M, Maffrand W, Le Fur G, Mukenge S, Ferla G. Functional assessment of neuronal cannabinoid receptors in the muscular layers of human ileum and colon. Dig. Liver Dis. 2002;34:262-269.
51 Mechoulam R, Hanus L. Cannabidiol: an overview of some chemical and pharmacological aspects. Part I: chemical aspects. Chem. Phys. Lipids 2002;121(1-2):35-43.
52 Meiri E, Thangiani H, Vredenburgh JJ, Barbato LM, Carter FJ, Yang HM, Baranowski V. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr. Med. Res. Opin. 2007;23(3):533-543.
53 Millan MJ. Descending control of pain. Prog. Neurobiol. 2002;66:355-474.
54 Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS, Jamison RN. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J. Pain 2008;9(3):254-264
55 Nurmikko TJ, Serpeil MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain 2007;133(1-3):210-220. 56 56 Petitet F, Jeantaud B, Reibaud M, Imperato A, Dubroeucq MC. Complex pharmacology of natural cannabinoids: evidence for partial agonist activity of A9-tetrahydrocannabinol and antagonist
activity of cannabidiol on rat brain cannabinoid receptors. Life Sci. 1998;63(1):PL1-6.
57. Pinsger M, Schimetta W, Voic D, Hiermann E, Riederer F, Pölz W. [Benefits of an add-on treatment with the synthetic cannabinomimetic nabilone on patients with chronic pain--a randomized controlled trial] [Article in German]. Wien
KIm. Wochenschr. 2006;118(11-12):327-335.
58. Plange N, Arend KO, Kaup M, Doehmen B, Adams H, Hendricks S, Cordes A, Huth J, Sponsel WE, Remky A. Dronabinol and retinal hemodynamics in humans. Am. J. Ophthalmol. 2007;143(l):173-174.
59. Redmond WJ, Goffaux P, Potvin S, Marchand S. Analgesic and antihyperalgesic effects of nabilone on experimental heat pain. Cun. Med. Res. Opin. 2008;24(4):1017-1024.
60. Roberts JD, Gennings C, Shih M. Synergistic affective analgesic interaction between delta-9tetrahydrocannabinol and morphine. Eur. J. Pharmacol. 2006;530(1-2):54-58.
61. Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005;65(6):812-819.
62. Rog DJ, Nurmikko TJ, Young CA. Oromucosal delta9-tetrahydrocannabinollcannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. Clin. Ther. 2007;29(9):2068-2079.
63. Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. 2006;Med. Hypotheses 66(2):234-46.
64. Sandrini G, Arrigo A, Bono G, Nappi G. The nociceptive flexion reflex as a tool for exploring pain control systems in headache and other pain syndromes. Cephalalgia 1993;13:21-27.
65. Seeling W, Kneer L, Büchele B, Gschwend JE, Maier L, Nett C, Simmet T, Steffen P, Schneider M, Rockemann M. [Delta(9)-tetrahydrocannabinol and the opioid receptor agonist piritramide do not act synergistically in postoperative pain] [Article in German]. Anaesthesist. 2006;55(4):391400.
66. Shakespeare DT, Boggild M, Young C. Antispasticity agents for multiple sclerosis (Cochrane Review). In: The Cochrane Library, Issue 3. Oxford: Update Software: 2003.
67. Siegling A, Hofinann HA, Denzer D, Mauler F, De Vry J. Cannabinoid CB(1) receptor upregulation in a rat model of chronic neuropathic pain. Eur. J. Pharmacol. 2001;415(1):R5-7.
68. Skrabek RQ, Galimova L, Ethans K, Perry D. Nabilone for the treatment of pain in fibromyalgia. J. Pain 2008;9(2):164-173.
69. Smith FL, Cichewicz D, Martin ZL, Welch SP. The enhancement of morphine antinociception in mice by delta9-tetrahydrocannabinol. Pharmacol. Biochem. Behav. 1998;60:559-566.
70 Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, Ko YD, Schnelle M, Reif M, Cerny T. Comparison of orally administered cannabis extract and delta- 9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-CachexiaStudy-Group. J. Clin. Oncol. 2006;24(21):33943400.
71 Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ 2004;329(7460):253.
72 Sylvestre DL, Clements BJ, Malibu Y. Cannabis use improves retention and virological outcomes in patients treated for hepatitis C. Eur. J. Gastroenterol. Hepatol. 2006;18(10):1057-1063.
73 Tomida I, Azuara-Blanco A, House H, Flint M, Pertwee RG, Robson PJ. Effect of sublingual application of cannabinoids on intraocular pressure: a pilot study. J. Glaucoma 2006;15(5):349-353.
74 Tseng AH, Harding JW, Craft RM. Pharmacokinetic factors in sex differences in Delta 9-tetrahydrocannabinol-induced behavioral effects in rats. Behav. Brain Res. 2004;154:77-83.
75 Verdejo-Garcia A, Lopez-Torrecillas F, Gimenez CO, Pérez-GarcIa M. Clinical implications and methodological challenges in the study of the neuropsychological correlates of cannabis, stimulant, and opioid abuse. Neuropsychol. Rev.2004;14:1-41.
76 Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. MuIt. Scier. 2004;10(4):434-441.
77 Wade DT, Makela PM, House H, Bateman C, Robson P. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. MuIt. Scier. 2006;12(5):639-645.
78. Wallace M, Schulteis G, Atkinson JH, Wolfson T, Lazzaretto D, Bentley H, Gouaux B, Abramson I. Dose-dependent effects of smoked cannabis on capsaicin-induced pain and hyperalgesia in healthy volunteers. Anesthesiology 2007;107(5): 785-796.
79. Ware MA, Ducruet T, Robinson AR. Evaluation of herbal cannabis characteristics by medical users: a randomized trial. Harm Reduct. J. 2006; 3:32.
80. Wilsey B, Marcotte T, Tsodikov A, Miliman J, Bentley H, Gouaux B, Fishman S. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J. Pain 2008; 9(6):506-521.
81. Wissel J, Haydn T, Muller J, Brenneis C, Berger T, Poewe W, Schelosky LD. Low dose treatment with the synthetic cannabinoid Nabilone significantly reduces spasticity-related pain: a doubleblind placebo-controlled cross-over trial. J. Neuroi. 2006;253(10):1337-1341.
82. Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn A, Thompson A; UK MS Research Group. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebocontrolled trial. Lancet 2003;362(9395):15171526.
83. Zajicek JP, Sanders HP, Wright DE, Vickery PJ, Ingram WM, Reilly SM, Nunn AJ, Teare U, Fox PJ, Thompson AJ. Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. J. Neurol. Neurosurg. Psychiatry 2005;76(12):1664-1669.
84. Zuardi AW, Shirakawa I, Finkelfarb E, Karniol 1G. Action of cannabidiol on the anxiety and other effects produced by delta-9-THC in normal subjects. Psychopharmacology 1982;76:245-250.












