An evaluation of the quality of medicinal grade cannabis in the Netherlands
| Articles - Cannabis, marijuana & hashisch |
Drug Abuse
Arno Hazekamp
Leiden University, Department of Pharmacognosy, Gorlaeus Laboratories, Einsteinweg 55, 2333CC Leiden, The Netherlands
Cannabinoids 2006;1(1):1-9
Abstract
Since 2003 medicinal grade cannabis is provided in the Netherlands on prescription through pharmacies. Growing, processing and packaging of the plant material are performed according to pharmaceutical standards and are supervised by the official Office of Medicinal Cannabis (OMC).
The quality is guaranteed through regular testing by certified laboratories. However, in the Netherlands a tolerated illicit cannabis market exists in the form of so-called ‘coffeeshops’, which offers a wide variety of cannabis to the general public as well as to medicinal users of cannabis. Since cannabis has been available in the pharmacies, many patients have started to compare the price and quality of OMC and coffeeshop cannabis. As a result, the public debate on the success and necessity of the OMC program has been based more on personal experiences, rather than scientific data.
The general opinion of consumers is that OMC cannabis is more expensive, without any clear difference in the quality.
This study was performed in order to show any differences in quality that might exist between the official and illicit sources of cannabis for medicinal use. Cannabis samples obtained from randomly selected coffeeshops were compared to medicinal grade cannabis obtained from the OMC in a variety of validated tests. Many coffeeshop samples were found to contain less weight than expected, and all were contaminated with bacteria and fungi. No obvious differences were found in either cannabinoid- or water-content of the samples. The obtained results show that medicinal cannabis offered through the pharmacies is more reliable and safer for the health of medical users of cannabis.
Keywords: medicinal grade cannabis, quality control, comparison, the Netherlands, Office of Medicinal Cannabis, coffeeshops.
Introduction
The use of cannabis as a medicine is increasingly becoming a topic of public discussion in a growing number of countries around the world. As a result of the United Nations Single Convention on Narcotic Drugs (1961), which was followed by a range of complementary treaties, international legislation has been a major obstacle for developments in this field for the last several decades. However, in recent years there have been some serious efforts to bring cannabis back into scientific and clinical research and to permit its use by medical patients. Initiatives that have been taken range from the decriminalization of medicinal cannabis use in the United Kingdom and Switzerland, to serious efforts to give patients direct access to high quality cannabis, or derivatives such as standardized extracts, like in Spain and Canada.
The Netherlands have become the world's first country to make herbal cannabis available as a prescription drug in pharmacies to treat a variety of patients. Since September 2003, pharmacies dispense medicinal cannabis to patients on prescription. Doctors practicing in the Netherlands are allowed to prescribe cannabis to treat a variety of indications (see below). As a general guideline, cannabis should be prescribed only after conventional treatments have been tried and found to be ineffective. As such, cannabis is effectively treated as a last-resort medication.
Because of the unique, liberal situation in the Netherlands with respect to drug laws, an illicit cannabis market can essentially openly compete with pharmacies, and experienced users of medicinal cannabis naturally compare both sources in terms of quality, medicinal effect, and price. It is therefore not surprising that opinions about the quality and efficacy of the stategrown cannabis emerged in the public media. Because of the popularity of cannabis as a theme in the media, opinions about the pharmacy product quickly found their way to the general public and it became clear that a group of medical cannabis users were not satisfied with the offered type of cannabis. A group of coffeeshop (see below) owners even started a campaign to promote the quality of their own material at the expense of the pharmacy cannabis. However, such opinions and initiatives were generally based on subjective measures and judgements by a group of authoritative and experienced users. Obviously, the opinion-based nature of this debate makes it complicated to evaluate the introduction of medicinal grade cannabis in the Netherlands and it clearly shows the need to address this matter in a scientific way.
The research presented here challenges the messages in the media about the dissatisfaction of some users with the medicinal grade cannabis offered by the Office for Medicinal Cannabis. This cannabis has been variously claimed to be too weak, too potent or too dry. According to some patients the ‘official’ cannabis doesn’t work, or it does so in a very different manner from what they are used to. Other users are wary of the treatment of medicinal grade cannabis by means of gamma-irradiation, which is routinely done in order to sterilize the material. The most common complaint, however, concerns the higher price. To address these complaints, we tested samples obtained from randomly selected coffeeshops according to the validated quantitative and microbiological analyses that are routinely used for quality control of medicinal grade cannabis in the Netherlands. The obtained data was compared with that of the simultaneously obtained pharmacy product. The tests for analysis of medicinal grade cannabis used in this study have been described in the official Dutch monography for medicinal cannabis.
The results presented in this study are intended as a contribution to the discussion about the necessity or advantage of having a policy of centrally regulated production and distribution of medicinal grade cannabis. We hope it can also help the users of medicinal cannabis to make a well-informed choice in the selection of their medicine.
The Dutch drug policy
In the current situation in the Netherlands, medicinal users of cannabis can obtain their cannabis material from two distinct sources: informally through the street market and formally through the pharmacy. To understand the choices that medicinal users in the Netherlands have to make in order to decide between these two sources, it is important to have some understanding about the Dutch drug policy concerning cannabis.
The basic principles of the Dutch drug policy were largely formulated in the mid-seventies. This policy does not moralise, but is based on the assumption that drug use is an undeniable fact and must be dealt with as practically as possible. The most important objective of this drug policy is therefore to prevent or to limit the risks and the harm associated with drug use, both to the user himself and to society. As a results of this, the Ministry of Health is responsible for co-ordinating drug policy.
The cornerstone of this policy is the law known as the Opium Act, which is based on two key principles. Firstly, it distinguishes between different types of
drugs on the basis of their harmfulness (cannabis products on the one hand, and drugs that represent an "unacceptable" risk on the other). The terms ‘soft-drugs’ and ‘hard-drugs’ refer to this distinction. Secondly, the law differentiates on the basis of the nature of the offence, such as the distinction between possession of small quantities of drugs intended for personal use, and possession intended for dealing purposes. Possession of up to 30 grams of cannabis is a minor offence, while possession of more than 30 grams is a criminal offence. Drug use itself is not an offence. This approach offers the scope to pursue a balanced policy through the selective application of criminal law.
Dealing in small quantities of cannabis, through the outlets known as “coffeeshops”, is tolerated (condoned) under strict conditions. There are currently about 700 such coffeeshops in the Netherlands, with the majority located in the bigger cities. Tolerance is a typically Dutch policy instrument which is based on the power of the Public Prosecutor to refrain from prosecuting offences. This principle is formulated in the law and is called the “expediency principle”. The small-scale dealing carried out in the coffee shops is thus an offence from a legal viewpoint, but under certain conditions it is not prosecuted. These conditions are: no advertising, no sales of hard-drugs, no nuisance must be caused in the neighbourhood, no admittance of and sales to minors (under the age of 18), and no sales exceeding 5 grams of cannabis per transaction. The stock of the coffeeshop should not exceed 500 grams of cannabis. If these rules are violated, the coffeeshop can be closed down by the municipal authorities.
The idea behind the Netherlands' policy towards the coffee shops is that of harm reduction. This is based on the argument that if small-scale cannabis dealing and use is not prosecuted under certain conditions, the users – who are mainly young people experimenting with the drug – are not criminalised (they do not get a criminal record) and they are not forced to move in criminal circles, where the risk that they will be pressed to try more dangerous drugs such as heroin is much greater. It is widely believed that drugs are legally available in the Netherlands, and that no effort is made to combat the supply side of the drug market. Nothing could be further from the truth. There is continual, intensive cooperation between the drug dependence care system,
the judicial authorities and the public administrators. With the exception of small-scale cannabis dealing in coffeeshops, tackling all other forms of drug dealing and production has high priority. The police and customs officials regularly seize large hauls of drugs and collaborate closely with other countries in the fight against organized crime. In 2000 alone, about 40,000 kg of cannabis and about 660,000 marihuana plants were seized and 1372 nursery gardens dismantled.
Tolerance does not mean that cannabis smokers can just light up a smoke anywhere they like outside a coffeeshop. Although no formal rules prohibit cannabis smoking in public places, such as bars, restaurants or concert halls, very few people do so. If they do, no sanctions are applied; but the person is likely to be asked by the personnel to put out the cigarette. The absence of formal regulations for the use of cannabis has opened the way for these informal norms, and their existence and effectiveness is an aspect of Dutch drug policy that is often underestimated and difficult to grasp by foreigners. For example, tourists who visit Amsterdam commonly make the mistake of thinking they can smoke cannabis 'everywhere'. It must be noted that the majority of the Dutch population, especially senior citizens, has never consumed cannabis and does not know much about cannabis regulations or habits. It’s in this complex situation of written and unwritten rules that consumers of medicinal cannabis in the Netherlands have to make choices about obtaining their medicine.
Medicinal cannabis in the Netherlands
Health Minister Els Borst (1994-2002) acknowledged the fact that a considerable group of people was using cannabis obtained through coffeeshops for medicinal purposes. However, its unofficial status makes it impossible to make any guarantees on the quality, consistency, or origin of the cannabis found in coffeeshops. In order to supply these patients with a safe and reliable source of high quality cannabis, the Office of Medicinal Cannabis (OMC) was established in March 2000 and started acting as a national agency on 1 January 2001. The OMC is the organisation of the Dutch Government which is responsible for the production of cannabis for medical and scientifical purposes. It holds the monopoly in the Netherlands for the import, export, and wholesale of this cannabis and its preparations on behalf of the Minister of Health, Welfare and Sport, and is notified to the International Narcotics Control Board (INCB) in Vienna. The previously mentioned United Nations Single Convention on Narcotic Drugs obliges the Netherlands to organize its Office in this way.
After an initial preparation period, medical grade cannabis became available in Dutch pharmacies in September 2003 on prescription only. Potential users must visit a medical professional (usually their own General Practitioner), who can grant approval for using cannabis for treatment in the form of a prescription.
Based on the availability and quality of clinical data and scientific literature, a selection of indications was made by the OMC for treatment with its medicinal grade cannabis. These are: nausea and loss of appetite resulting from chemotherapy, radiotherapy or HIV combination therapy; palliative treatment for cancer and HIV patients; spasticity and pain associated with multiple sclerosis or spinal cord injury; chronic neurogenic pain; and physical or verbal tics caused by Tourette's syndrome. However, if they find it necessary in selected cases, medical professionals are allowed to prescribe cannabis for other indications as well.
The medicinal grade cannabis comes in the form of dried and manicured flowertops of female plants and is produced by an authorized grower (Bedrocan BV, Veendam, the Netherlands). Plants are cultivated indoors according to guidelines that have been derived from the general rules for Good Agricultural Practise of the Working Group on Herbal Medicinal Products of the European Medicines Evaluation Agency (EMEA) [3]. The detailed specifications for medicinal grade cannabis can be found on the website of the OMC [15].

Figure 1: The 5 gram package of medicinal grade cannabis
as currently available in Dutch pharmacies. There are
currently 2 varieties available; the variety shown is
‘Bedrocan’ which has a mean THC content of 18%. (Not
shown is the variety ‘Bedrobinol’, with a mean THC content
of 13%).
Materials and methods
Medicinal cannabis of the OMC Currently, two different cannabis varieties are available in Dutch pharmacies: Bedrocan, mean THC content 18% (specifications: 15.5-21.0%) and Bedrobinol, mean THC content 13% (specifications: 11.0-14.8%). The product is finally packaged in sealed plastic containers in quantities of 5 grams for distribution (figure 1). For this study, two original pharmacy packages (total 10 grams) of each variety were obtained through the OMC.
Cannabis sampling
In order to conduct a statistically acceptable experiment on the quality of cannabis obtained from coffeeshops, 10 different coffeeshops were visited. These were randomly and independently selected by Intraval (Groningen/Rotterdam, The Netherlands). Furthermore, an unofficial Dutch foundation specialized in providing cannabis to medical patients was included in the study, resulting in a total of 11 locations where samples were collected. In order to guarantee that these locations remain anonymous, locations are identified by letters only (A-K). In order to limit traveling time, only coffeeshops in the West and middle of the Netherlands (the provinces of Zuid-Holland, Noord-Holland and Utrecht) were visited. About 70% of all Dutch coffeeshops are located in this most densely populated region of the Netherlands [18].
The person that visited the coffeeshops for collection of the samples pretended to be a family member of a patient suffering from multiple sclerosis, and asked what type of cannabis was recommended for this indication. The recommended cannabis was then purchased (10 grams) for performing the study.
Determination of cannabinoid composition and water content
In order to compare the potency of the samples, contents of delta-9-tetrahydrocannabinol (THC) and its acidic precursor tetrahydrocannabinolic acid (THCA) were determined by HPLC analysis. For the analysis, we used the validated HPLC-method as described in the official Dutch monography for medicinal cannabis [3]. In order to confirm the results obtained by HPLC, quantification of THC and THCA was repeated by using a recently developed quantitative 1H-NMR method [6].
Although THC is known to be the major active compound in the cannabis plant, it is widely believed by researchers, as well as patients, that other components (predominantly the cannabinoids) also could play a role in the medicinal properties of cannabis [22]. The bioactivity of such compounds has been shown in a large variety of scientific studies. Examples are the cannabinoid cannabidiol (CBD) that was shown to be active in the reduction of neuropathic pain [14] and cannabinol (CBN) that acts on the immune system [8]. To include non-THC type cannabinoids in our evaluation, the total
profile of cannabinoids present in each sample was measured by HPLC, as described above, and by gas chromatography (GC) [7].
Water content of the samples was determined according to the method of Karl-Fischer and was expressed as % of sample weight. Obtained values were confirmed by determining loss on drying after 24 hours heating at 40ºC under vacuum.
Microbiology
Policy of the OMC prescribes that microbiological analysis of the medicinal cannabis must be performed after the plants are harvested and again after the finalproduct is packaged. Packaged material must conform with the European Pharmacopoeia (EP), chapter 5.1.4, category 2: “microbiological quality of pharmaceutical preparations”, which deals with the requirements for medicinal preparations for inhalation. To prevent the formation of microbial toxins, the product is sterilized shortly after harvest by gamma-irradiation (dose <10 kGy) and subsequently packaged under aseptic conditions. If the packaged product does not conform to the microbiological specifications of the EP, the entire batch is rejected for further medical use.
In order to determine the level of microbiological contamination of the obtained samples, microbiological analysis for the presence of potentially harmful bacteria and fungi was performed by Bactimm BV (Nijmegen, The Netherlands), the company that also performs the routine analyses of medicinal cannabis for the OMC.
Price
The most relevant way to compare prices of medicinal preparations is by expressing the price relative to the amount of active ingredient present (price per dosage). In the case of medicinal use of cannabis, it is widely assumed that the major active constituent is THC, although other cannabinoids are believed to play a role as well. Therefore, prices were corrected for the obtained weight of the samples as well as their content of THC. Corrected prices were expressed per 100 mg of THC.
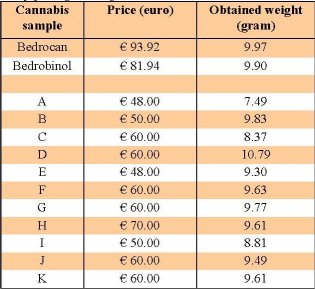
Table 1: Prices paid for each sample when ’10 grams’
was demanded, and amount of sample (in grams) actually
obtained in the purchase. For Bedrocan and Bedrobinol,
‘10 grams’ was obtained by combining 2 standard pharmacy
packages of 5 grams each.
Results and discussion
For completion of all the analytical tests, 10 grams of cannabis was needed, but the Dutch policy concerning the toleration of coffeeshops prohibits selling more than 5 grams per client per transaction. Therefore in most cases the sample collector had to return at a later time to obtain another 5 grams of the same cannabis. However, in 4 out of 11 visits the collector was allowed by the coffeeshop to obtain 10 grams at once. The workers in most coffeeshops were found to have experience answering questions concerning the medicinal use of cannabis and were willing to offer advice on matters such as method and frequency of use, as well as on expected results. Although the cannabis was explicitly purchased for medical use, none of the visited locations asked to see a doctor’s prescription before selling the cannabis.
Obtained samples were weighed in order to divide them up in portions for performing the different tests. It was found that less than 9.50 grams were present in the obtained package(s) in 5 out of 11 cases, meaning a deficit of more than 5%. A variation of 5% in content is the tolerance that is usually accepted in trade in the EU. In one case (coffeeshop A) only 7.49 grams (- 25%) were delivered. Although it was not an objective of our study, these results indicate that falsification of weight (whether intentionally or not) is not merely an incidental problem. In contrast, both samples obtained from the OMC contained almost exactly the expected amount of 10 grams (± 0.1 gram). The prices and obtained weights of the samples are listed in table 1.
In fresh cannabis plant material, THC is predominantly present in the form of its acidic precursor THC-acid (THCA). Under the influence of heat or storage, THCA can be converted into free THC. For the recreational as well as the medicinal user, THC is the most important bio-active component, and therefore it is common practise in analytical laboratories to determine the total THC content of cannabis (THCA + THC) after heating of the plant material. However, this method is not completely reliable because a full conversion of THCA to THC is difficult to achieve. Furthermore, during the heating process degradation products of THC (such as cannabinol or delta-8-THC) can form or evaporation of THC can occur [19]. During this study these problems were prevented by determining the amount of THCA and THC individually. From these results the total THC content was then calculated. This method has
only recently become available, through the development of a reliable THCA reference standard for quantification [5,16].
The THC-content of samples is shown in figure 2. For all coffeeshop samples, the THC content was found to be in the relatively narrow range of 11.7-19.1% (as percentage of dry weight plant material). The THC content of the pharmacy varieties fell also within this range: variety ‘Bedrocan’ (16.5% THC) was found in the middle of the range, while variety ‘Bedrobinol’ (12.2% THC) was at the lower end of the range.
Besides THC and THCA, other cannabinoids were taken into account as well during analysis of the cannabinoid composition of the samples. However, no major differences were observed among the coffeeshop samples when comparing the obtained GC- or HPLCchromatograms. Likely, this is the result of decades of cross-breeding and selection for high-THC producing strains of cannabis. This process has minimized the variability between the cannabis strains, with some exception for their content of THC. Some representative HPLC chromatograms are shown in figure 3.
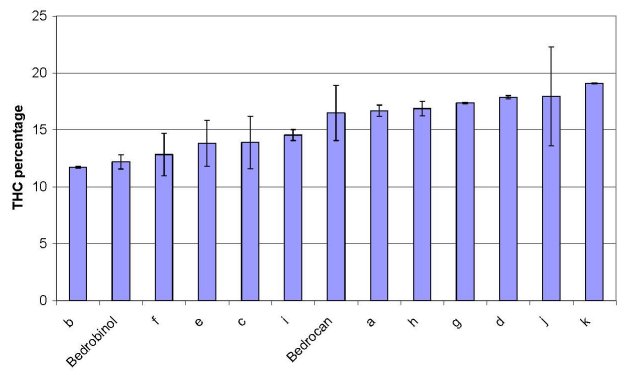
Figure 2: Content of total THC for each sample in % of sample weight. Results are shown in increasing order. Values are the mean
of 2 determinations. Errorbars indicate standard error.
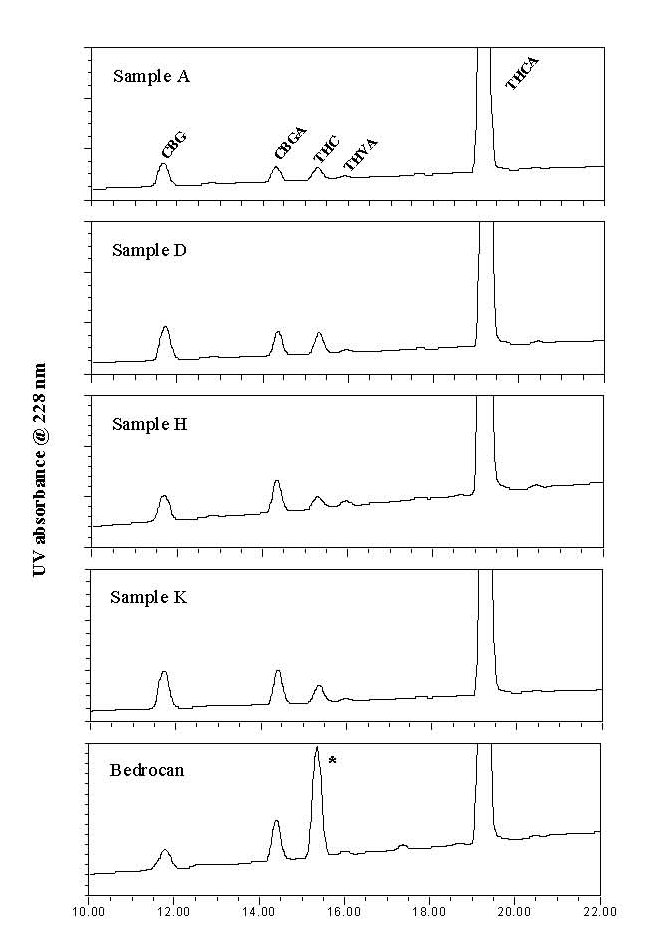
Figure 3: HPLC chromatograms (228 nm) of selected samples.
No cannabinoids were observed outside the shown
region of the chromatograms. Pharmacy cannabis contains a
larger proportion of free THC (*). CBG: cannabigerol;
CBGA: cannabigerolic acid; THVA: tetrahydrocannabivarinic
acid.
When coffeeshop samples were compared to the OMC samples, only one noticeable difference was observed: the latter contains a larger proportion of free THC, and therefore a lower proportion of its carboxylic acid precursor THCA. We expect this to be the result of handling and packaging, which is likely to convert some THCA into free THC. A higher content of free THC can be beneficial when a patient consumes the cannabis in a form that has not been heated strongly or long enough, like in the case of an infusion (for cannabis tea). Under such conditions THCA will not be completely transformed into THC so a smaller amount of the active component THC will be consumed. However, when the cannabis is consumed by smoking or in the form of strongly heated products (e.g. baked products such as cookies), the transformation of THCA into THC will be virtually complete and the observed differences in initial free THC content will become irrelevant.
When water content of the samples was compared, it was found that the OMC-variety ‘Bedrocan’ (water content 4.7%) was not significantly different compared to the coffeeshop samples, where water contents ranged from 3.9-5.5%. For the variety ‘Bedrobinol’ however, a significantly higher water content of 8.0% was found. According to the OMC, this value was intentionally higher, after comments from users, in order to make the inhalation of this variety more pleasurable. According to OMC specifications the water content of the cannabis at the time of quality control (directly after packaging) must be between 5-10%.
The EP requirements with regard to microbiological purity for inhalation preparations set the following limits for sample contamination: total molds and aerobic bacteria: ≤10 colony forming units (CFU) per gram; total enterobacteria and gram-negative bacteria: ≤100 CFU per gram. The infectious bacteria Pseudomonas aeruginosa and Staphylococcus aureus must be completely absent. As shown in table 2, all samples obtained from coffeeshops carried contamination levels of bacteria and/or fungi above these limits. In contrast, both cannabis varieties from the OMC were found to be clear of such contaminations. According to the OMC, rejection of its medicinal cannabis based on microbiological contamination has never occurred to date.
The mycological laboratory of Centraal Bureau voor Schimmelcultures (CBS, Utrecht, the Netherlands) further analyzed the contaminants present in one of the samples (sample K), and identified several known pathogens, including the intestinal bacterium Escherichia coli, and fungi of the Penicillium,
Cladosporum and Aspergillus types. Some of these microbes are capable of producing hazardous mycotoxins, such as aflatoxin B, ochratoxin A and B, and sterigmatocystine.
Aflatoxins, in particular, are known to be extremely potent carcinogens [17]. They are not completely destroyed by heat during smoking, and thus may be inhaled [2,10]. The presence of potentially hazardous fungi on recreationally-used cannabis has been described routinely and increasingly these fungi are being acknowledged as an underestimated source of neurological toxicity [1] or infections such as aspergillosis [4,11,20]. There are some indications that the use of anti-inflammatory steroids can increase the susceptibility to fungal infections [12] and it should be noted that a significant fraction of the population of patients that uses medicinal cannabis also uses such drugs. Moreover, medicinal cannabis is relatively commonly used by HIV/Aids patients and other types of patients that, because of their compromised immune systems, are specifically vulnerable to infections. Opportunistic
lung infections with Aspergillus have already been suggested as a serious contribution to morbidity in this subgroup of patients [9,20].
Even for consumers who are not immunocompromised, neurological toxicity of contaminated cannabis samples is pointed out as a health risk [1].
Therefore, these combined data indicate that medicinal use of cannabis that has been purchased from uncontrolled sources could be considered as a potential health risk for the population of medicinal users, particularly for those who consume larger amounts of cannabis on a daily basis.
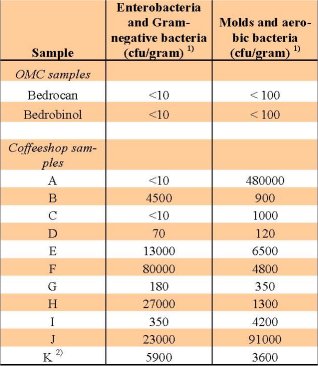
Table 2: Presence of bacteria and fungi (in cfu per gram)
in the studied samples.
1) CFU per gram = colony forming units present in one
gram of the sample. 2) The contaminants on sample K
were further identified to be the bacterium E. coli, and
fungi of the types Penicillium, Cladosporum and Aspergillus.
The higher price of medicinal cannabis has proven to be a major drawback for medical patients in the Netherlands to obtain their cannabis from pharmacies. By expressing the price of the samples relative to the level of THC present, a fair comparison between the obtained samples is possible. Results are shown in figure 4. It is shown that the price of the pharmacy variety ‘Bedrocan’ (€ 5.72) is somewhat above the range of prices that were paid for coffeeshop samples (€ 3.11– 5.16). The relative price of the ‘Bedrobinol’ variety, however, is significantly higher (€ 6.80). According to OMC, the higher costs of medicinal grade cannabis are the result of maintaining a high quality standard for the product. Included are: production according to pharmaceutical standards, aseptic packaging, distribution and costs made by pharmacies. Moreover, costs accrue as a result of constant quality controls and microbiological analyses. Finally, pharmacy cannabis includes a 6% VAT charge, while the EU VAT system does not allow that VAT is charged on the illicit (although tolerated) cannabis from coffeeshops.
Conclusion
The simple rules of supply and demand usually result in the consumer buying the product with the best quality-to-price ratio. Because of such forces, the unique situation in the Netherlands has led to a confusing situation for medicinal users of cannabis. Price comparisons and superficial inspection easily leads to favouring the cheaper material from the coffeeshops over the more expensive, but seemingly equal, pharmacy grade. The fact that only the quality of the latter is guaranteed through regular controls does not seem to impress most consumers. However, it is obvious that the standards for any medicinal preparation are high and that these can be enforced only by appropriate analytical testing. According to the OMC, another reason why the price of Cannabis available in pharmacies is currently somewhat higher than expected, is because sales are relatively low. If the number of patients would increase, this could influence the price because the fixed costs per sold unit would drop.
Because the number of coffeeshop samples that were used for this study was limited, conclusions must be drawn with some precaution and results presented here should be reported as incidental findings. Still, based on the obtained results we concluded that the price paid for medicinal cannabis distributed through the Dutch pharmacies must be considered reasonable. The cannabinoid strength and composition of the pharmacy products and the water content are not significantly different from other types of cannabis. In contrast, the pharmacy product is guaranteed to have a consistent potency, and potentially dangerous contaminations are absent. These results indicate that routine analysis of the cannabis results in a significantly safer product of
high and reproducible quality. Delivery of medicinal cannabis to patients through the OMC and pharmacies results in a reliable product without the health risks commonly associated with coffeeshop cannabis.
Some patients have claimed that the official cannabis simply is not as good as their personal choice of ‘mediweed’. Certainly, the possibility remains that cannabis varieties with a similar cannabinoid profile can have different strengths or effectiveness, based on the presence of other components such as terpenoids or flavonoids. Nevertheless, the current scientific consensus is that it is mainly the cannabinoids that are responsible for the bioactivity of cannabis, and testing of the samples by two different methods did not show obvious differences in cannabinoid composition. In conclusion, it seems that there remains some room for discussion on this point.
When patients choose to obtain cannabis from an uncontrolled source, they must realize that they do so with a certain risk to their health. In this test, we did not check for the presence of pesticides, fungicides or heavy metals, but there are plenty of indications that these are frequently present in cannabis samples from uncontrolled sources [13,21]. The same lack of quality control makes it impossible to determine whether products that are claimed to be grown organically, like in some coffeeshops, are really that much more trustworthy. Ultimately, it is the consumer that makes the choice. We hope that the research presented in this article may help the consumer to make an informed and safe choice.
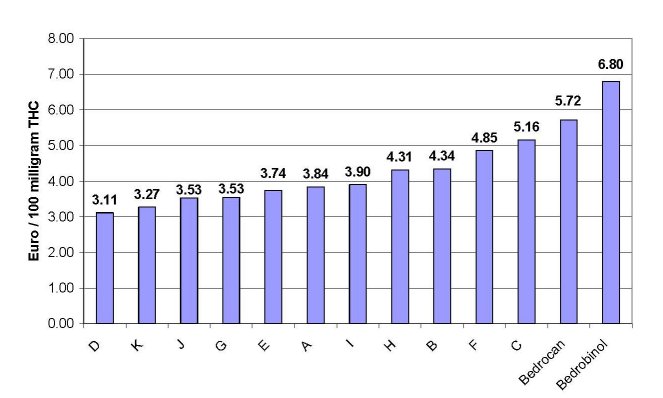
Figure 4: Price of each sample, expressed as price (in euros) paid per equivalent of 100 mg THC. Results are shown in increasing
order.
Tests for the presence of heavy metals and pesticides are routinely performed for the OMC cannabis. Therefore the medicinal grade cannabis in Dutch pharmacies is guaranteed to be free (below official standard limits) of such contaminants. Unfortunately, because such tests are very costly, they could not be carried out as part of this study. Future studies should therefore include a larger number of sampled locations, and could include analysis for the presence of heavy metals, pesticides or fungicides.
Acknowledgements
Pieter Seijrier is gratefully acknowledged for his help in performing this study.
References
1. Carod Artal FJ. Neurological syndromes associated with the ingestion of plants and fungi with a toxic component (II). Hallucinogenic fungi and plants, mycotoxins and medicinal herbs. Rev Neurol. 2003;36(10):951-960.
2. Georggiett OC, Muino JC, Montrull H, Brizuela N, Avalos S, Gomez RM. Relationship between lung cancer and aflatoxin B1. Rev Fac Cien Med Univ Nac Cordoba. 2000;57(1):95-107.
3. Guidelines for cultivating cannabis for medicinal purposes; Annex to the regulation of the Minister of Health, Welfare and Sport of 9 January 2003. GMT/BMC 2340685 [cited 2006 July 08]. Available from: http://www.cannabisbureau.nl/pdf/GAP_EN_2003-01-07.pdf.
4. Hamadeh R, Ardehali A, Locksley RM, York MK. Fatal aspergillosis associated with smoking contaminated marijuana, in a marrow transplant recipient. Chest. 1988;94(2):432-433.
5. Hazekamp A, Choi YH, Verpoorte R. Quantitative analysis of cannabinoids from Cannabis sativa using 1H-NMR. Chem Pharm Bull. 2004;52 (6):718-721.
6. Hazekamp A, Peltenburg A, Verpoorte R, Giroud C. Chromatographic and Spectroscopic Data of Cannabinoids from Cannabis sativa L. J Liq Chrom Rel Technol. 2005;28(15):2361-2382.
7. Hazekamp A, Simons R, Peltenburg-Looman A, Sengers M, van Zweden R, Verpoorte R. Preparative isolation of cannabinoids from Cannabis sativa
by centrifugal partition chromatography. J Liq Chrom Rel Technol. 2004;27(15): 2421- 2439.
8. Jan TR, Rao GK, Kaminski NE, Cannabinol enhancement of interleukin-2 (IL-2) expression by T cells is associated with an increase in IL-2 distal nuclear factor of activated T cell activity. Mol Pharmacol. 2002;61:446-454.
9. Johnson TE, Casiano RR, Kronish JW, Tse DT, Meldrum M, Chang W. Sino-orbital aspergillosis in acquired immunodeficiency syndrome. Arch Ophthalmol. 1999;117 (1):57-64.
10. Kagen SL, Kurup VP, Sohnle PG, Fink JN. Marijuana smoking and fungal sensitization. J Allergy Clin Immunol. 1983;71(4):389-393.
11. Llewellyn GC, O’Rear CE. Examination of fungal growth and aflatoxin production on marihuana. Mycopathologia. 1977;62(2):109-112.
12. Marks WH, Florence L, Lieberman J, Chapman P, Howard D, Roberts P, Perkinson D. Successfully treated invasive pulmonary aspergillosis associated with smoking marijuana in a renal transplant recipient. Transplantation. 1996;61
(12):771-1774.
13. Mc Partland JM, Pruitt PL. Medical marijuana and its use by the immunocompromised. Altern Ther Health Med. 1997;3(3):39-45.
14. Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, Sansom C. Initial experiences with medicinal extracts of cannabis for chronic pain: Results from 34 'N of 1' studies. Anaesthesia. 2004;59(5):440-452.
15. Office of Medicinal Cannabis, the Netherlands. Available from: http://www.cannabisbureau.nl.
16. Farmalyse BV, Zaandam, the Nethelands; Producer of cannabinoid standards. Available from: http://www.bactimm-bv.com/farmalyse.
17. Ricordy R, Gensabella G, Cacci E, AugustiTocco1 G. Impairment of cell cycle progression by aflatoxin B1 in human cell lines. Mutagenesis. 2002;17(3):241-9.
18. Snippe, J, Bieleman B, Naayer H, Ogier C. Preventieve doorlichting cannabisbranche c.a.. St. Intraval, Groningen-Rotterdam. 2004.
19. Veress T, Szanto JI, Leisztner L. Determination of cannabinoid acids by high-performance liquid chromatography of their neutral derivatives formed by thermal decarboxylation in an open reactor. J Chromatogr. 1990;520:339-347.
20. Wallace JM, Lim R, Browdy BL, Hopewell PC, Glassroth J, Rosen MJ, Reichman LB, Kvale PA. Risk factors and outcomes associated with identification of Aspergillus in respiratory specimens from persons with HIV disease. Pulmonary Complications of HIV Infection Study Group. Chest. 1998;114:131-137.
21. Ware MA, Tawfik VL. Safety issues concerning the medical use of cannabis and cannabinoids. Pain Res Manage. 2005:10(Suppl A):31A-37A.
22. Williamson EM, Evans FJ. Cannabinoids in clinical practice. Drugs. 2000;60(6):1303-1314.












