Options for regulating new psychoactive drugs: a review of recent experiences
| Articles - Various general |
Drug Abuse
Forword
This paper is intended to provide the basis for a discussion of policy options in dealing with new psychoactive substances that show signs of popularity and of harmfulness within a wider project being undertaken by the UK Drug Policy Commission and Demos, the outcomes of which are presented in Taking Drugs Seriously: a Demos and UK Drug Policy Commission report on legal highs (Birdwell et al., 2011).
The review considers the international context, current approaches to tackling new drugs with a main focus on the USA and Europe, and examines four specific examples of new drugs: BZP, Spice, mephedrone and naphyrone. It also looks at the possible learning from other regulatory frameworks, those for foodstuffs and for alcohol and tobacco.
When these drugs come to public attention, very little is known about the possible harms to users or whether they are complements or substitutes for other dangerous intoxicants. Literally nothing is known about the harms that might be generated by an illicit market if the drug were prohibited. The paper highlights the dilemmas associated with making regulatory decisions with such limited information. In recent years, great attention has been given to the “precautionary principle” as a guideline for policy making under uncertainty. However the complication here is that giving deference to only the possible harms faced by the individual user ignores the more subtle, but still real, harms that arise from the decision to prohibit. No consideration is currently given to any of the perceived benefits of use, which must play a part in an individual’s decision to use drugs. However, any kind of implicit legalization also may have unintended consequences, such as encouraging greater use.
The report concludes that there is considerable unease with the existing system for making decisions about newly emerging psychoactive substances. Of fundamental concern is the fact that there is an overriding bias in the decision-making process towards the prohibition of new psychoactive substances, but achieving a more balanced set of regulatory decisions is a major task. The existing system has worked well in the sense that no major problem has emerged from a decision to allow into commerce a drug that turns out to be dangerous, the burden of proof for creating an alternative system is thus a heavy one. A research effort to improve understanding of the substitution effects of new entities, as well as an examination of how prohibition of these entities creates market harms, would go a long way toward clarifying the issues.
1. INTRODUCTION
It is easy to think of the drug problem as defined by a few substances that have troubled societies for decades, if not centuries. Heroin, still regarded as the drug causing the greatest harm in aggregate, has been part of the consciousness of most Western societies for a century. For the United Kingdom heroin has been a serious health and crime problem for at least the last forty years. Cocaine, now comparably important, has emerged only in the last decade in the UK as a large-scale problem but it had been well known through its ravages in the US for twenty years before that.
However at the edges of the problem, there have emerged new psychoactive substances, not covered by the existing system of drug-specific regulations and prohibitions; two examples that have been prominent recently are mephedrone and Spice. Many, but not all, of these substances are the creation of entrepreneurial chemists. Some are natural substances, for which new and more dangerous modes of ingestion have been developed or whose intoxicating properties have not previously been understood. Yet others are manufactured substances for which also new uses as intoxicants have been found. A wide range of terms have been used to describe such substances. These include legal highs, synthetics, research chemicals, designer drugs and party drugs, all of which are within the scope of this review.
The problem is not an entirely new one. Late 20th century chemistry was advanced enough to produce a rapid flow of new psychoactive drugs that found their niches in recreational markets (e.g. ketamine and GHB). Alexander Shulgin, a prominent chemist in the development of new psychedelics in the United States, notes that there were only two such substances in 1900 (marijuana, mescalin), 20 by 1950 and over 200 by 2000 (quoted in Kau 2008, pp.1079-1080). Governments have struggled throughout the last century with how to respond to these new entities about whose effects they were poorly informed, generally prohibiting them for precautionary reasons. The number of drugs on the list banned by international conventions has risen sharply, very much as Shulgin sketched for psychedelics.
When the Single Convention passed in 1961 there were 85 prohibited drugs, whereas by 1995 there were 282 (Babor et al., 2010). What is striking is how narrow and/or ephemeral are the niches that these new drugs have so far occupied in the recreational market. Even LSD, perhaps the most venerable of them, has almost disappeared in the US, after 40 years, following a major enforcement success in the year 2000 (Grimm, 2009).2 Others simply lose popularity, either because that particular experience is unattractive to a new generation or because of fears about adverse effects, usually reflecting actual experience of recreational users rather than government announcements3.
Though the problem itself is demonstrably not new, it is easy to identify factors that may make it more acute today: the growing speed with which these new entities can be developed; the availability of efficient and difficult-to-regulate distribution systems via the internet; and perhaps Western society’s greater tolerance for altered states of consciousness. The difference between a “good” high (alcohol in moderation) and a “bad” one (Ecstasy) is becoming harder to explicate.
What is striking is just how modest and localized this problem has been so far. Though the EMCDDA notes that the number of substances on the early warning list, for which assessments are required, has risen in recent years and was 24 in 2009, almost double the 2008 figure (Europol and EMCDDA, 2010), most of those substances turn out to have created very little problem at the time that they enter the list4. Often the problem is almost exclusively in one European nation, as in the UK for mephedrone. In the United States, where this problem is usually described colloquially as “designer drugs”, a term that flatters the producers (Kau, 2008), the number of new psychoactive substances is also very small. Coulson and Caulkins (2011) identify just 73 new substances that have been subject to scheduling
decisions since 1971 in the United States. Intriguingly, the substances that have been most prominent in Europe have been essentially unknown in the United States. For example, a 2010 search of the long list of entities recorded in the Drug Abuse Warning Network5 found no mention of any of BZP, Spice6, mephedrone, and naphyrone, using both street and technical names.
Unsurprisingly, the policy analytic literature on this topic is tiny. The policy analysis that is available is focused on specific substances or else is very legal and bureaucratic in orientation. Winstock and Ramsey (2010) is a notable exception that contains a number of important observations about the regulatory dilemmas posed by these substances.
This paper is intended to provide the basis for a discussion of policy options in dealing with new psychoactive substances that show signs of popularity and of harmfulness before they have been subject to full regulatory scrutiny under the domestic and international drug-specific regulations. Are there options that effectively control the risk of widespread use of potentially dangerous substances without incurring the dangers of prohibition and accompanying black markets?
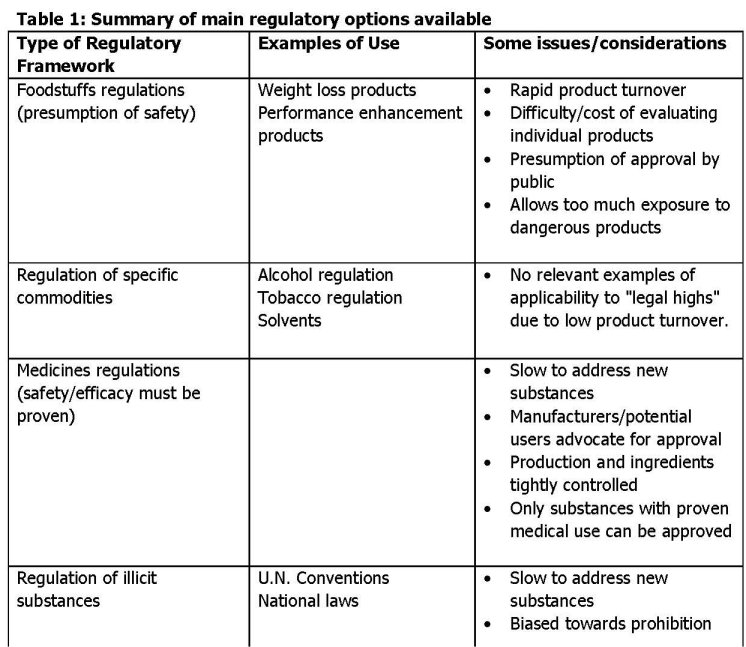
There are essentially four main frameworks that are used to regulate the use and availability of substances that may be consumed by humans. These are, in ascending order of restrictedness:
1. Foodstuffs regulations
2. Regulations relating to specific commodities, such as tobacco and alcohol, but also substances with other uses, such as solvents.
3. Medicines regulations
4. The regulation of illicit substances covered by international conventions and related national and domestic legislation
To date governments have largely limited their considerations to regulation of new substances under the illicit substances frameworks. The next section describes the current system of classifying psychoactive substances and how it deals with new substances in the UK, European Union and the United States. That is followed by a review of how various countries responded to four specific new drugs that have received considerable attention: BZP, Spice, mephedrone and naphyrone. That is then followed by a discussion of the relevant experiences with other regulatory approaches. The final section offers an assessment of the current systems and the dilemmas faced by modern governments in dealing with this phenomenon.
The paper’s principal substantive conclusion is that there is an inherent, perhaps inescapable, bias in the system towards prohibiting new substances about which little is known. The negative consequences to decision makers of permitting on the market, in any way, a drug that later turns out to be dangerous are very high. The negative consequences to decision makers of keeping off the market a drug that is in fact harmless, even if the resulting prohibition worsens the problems related to that drug, are minimal. Innovative regulatory schemes do not have much promise of cutting this Gordian knot, unless the public can be persuaded to view the pleasures or other benefits from these substances as potential gains to society, which itself may be considered a questionable goal.
2. CURRENT APPROACHES TO DEALING WITH NEW SUBSTANCES
This problem, labeled as “legal highs”, has in recent years attracted considerable official attention and even more media coverage. The regulatory system involves international, national and sub-national institutions and laws. I describe here some aspects of the systems of the United Nations (WHO), the European Union, and two nations: the United Kingdom and the United States; other nations are mentioned as needed.
THE INTERNATIONAL SYSTEM
The United Nations
Two of the mainstays of the international drug control system, the 1961 Single Convention and the 1971 Convention on Psychotropic Drugs, provide for a process to add new drugs to the list of those that are subject to supervision. An Expert Committee on Drug Dependence, consisting of international experts, is operated by the World Health Organization. Once the Expert Committee has decided that a substance should be subject to scheduling, i.e. some degree of regulation, that recommendation is considered by the Commission on Narcotic Drugs, a set of 43 nations chosen by the UN Economic and Social Council. Once approved by the CND, all Member States (essentially all nations) must adopt a scheduling decision at least as stringent as that. The process is a slow one, the Expert Committee meeting only approximately every two years during the last decade, lasting for only 3-5 days and handling just a small number of substances at each meeting. There is no counterpart to the emergency procedures that have been adopted by various national systems.
The European Union (EU)
The EU in 2005 promulgated Council Decision 2005/387/JHA7 which created a set of procedures for dealing with new psychoactive substances that threatened to become popular with large numbers of users and with potential serious consequences to individual users. The procedures are comprehensive, covering the whole process from detection through risk assessment to legal action. They are central to this study, so I provide a relatively lengthy excerpt from the Decision:
“For risk assessment the Decision had five elements:
• An assessment of risks caused by use of, manufacture of, and traffic in a new substance, as well as the potential involvement of organised crime;
• The risks to be assessed include health and social risks, as well as the consequences of possible control measures;
• The assessment is based on the analysis of scientific data and law enforcement information, made available by, e.g. health, social and law enforcement sources (but not necessarily limited to these);
• The assessment may or may not take into account the same factors which warrant the placing of a substance under international control;
• The assessment may be done in accordance with a formalised (legally based) procedure and it may be carried out by a scientific or expert body.” (Hughes and Blidaru, 2009)
A good depiction of the process indicating its procedural and bureaucratic complexity is provided in Figure 1, taken from Sedefov and Lopez (2009)..Many European institutions are involved and the process is time consuming.
Figure 1. Representation of EU Council decision making
(Source: Sedefov and Lopez, 2009)
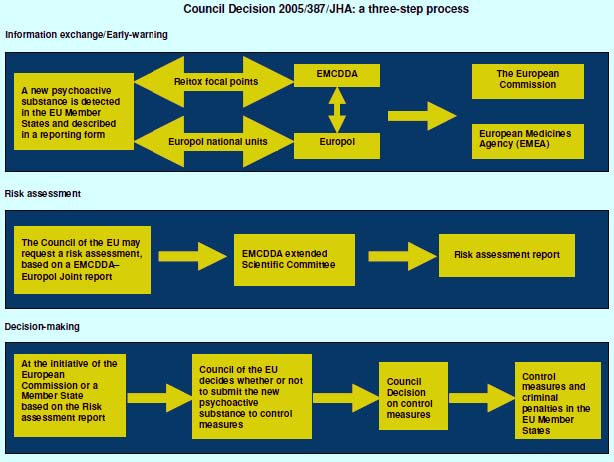
These EU procedures point to the difficulty of an assessment soon after a substance has entered the market. For example, the risk assessment criteria include potential organized crime involvement (criterion 1) but provide no guidance as to how this might be done. There are two problems in assessing organized crime involvement, mirrors of each other. On the one hand, if the drug were made explicitly legal, organized crime might be replaced by legitimate manufacturers who were initially unwilling to produce and market a substance not yet dealt with in the formal regulatory system. On the other hand, it is also possible that organized crime may enter once prohibition is enacted, since prohibition excludes the marginal operators who are willing to produce/distribute when the product’s status is ambiguous but not when it is clearly illegal. Analytically it is extremely challenging to make projections of the effects of legalization, since they have not been done before for any drug. It may in principle be possible to identify the factors (production process, users, intensity of enforcement etc.) most likely to lead to organized crime involvement and those that discourage legitimate manufacture during the period when the status is uncertain. However, for the foreseeable future this phenomenon is not susceptible to systematic analysis.
Another problem in implementing this approach is that the assessment of health risks is essentially impossible at the stage the decision is called for. This is discussed in detail below.
NATIONAL SYSTEMS
Individual EU Member States have their own regulatory systems for dealing with emerging substances, as reviewed by Hughes and Blidaru (2009). The basic model is to add substances to an existing list of those that can either not be marketed at all or which can only be sold under specific controls. There is variation in whether this requires legislative action or can be done by an executive agency. Some nations use a generic system for scheduling, whereby a group of related substances are banned8, rather than requiring that each of the substances be considered and listed separately. Still other nations use an analogue system, which “addresses more general aspects of similarity in chemical structure to a ‘parent’ compound; this aspect might be supplemented by a requirement for similarity in pharmacological activity, attempting a more specific delineation of the analogue system’s sphere of control.” (Hughes and Blidaru, 2009, pp6-7). Some nations allow for accelerated scheduling decisions, based on a declared emergency with respect to a specific drug.
Few of the systems produce analytical reports9. Apart from those provided by the UK Advisory Council on the Misuse of Drugs, those reports that were available for review are of limited conceptual sophistication. Consider for example Ireland, which has been active in the field. It has published an analysis of regulatory options for head shops, which are prominent there.10 The assessment makes no mention of any potential adverse effects of prohibition. It identifies the dangers of not regulating and the potential gross gains of the regulatory options. The only negative aspects of regulation that are given any attention are the costs of operating the regulation. It is naïve compared to, for example, environmental regulatory analysis, which requires much more careful balancing of costs and benefits of each option11.
The United Kingdom
The United Kingdom has a complex system of classifying drugs; it involves two separate scales: Classes and Schedules (Home Office, 2006). The Classes (A, B and C) refer to substances that are subject to the 1971 Misuse of Drugs Act (as modified). The class reflects their relative harm and the maximum length of sentences for offenses of use and distribution involving the drug. The five-part Schedule reflects the utility of the drug for medical purposes and the level of control that must accompany its distribution. Schedule I is no allowed medical use (e.g. LSD, Ecstasy, cannabis). Schedule II includes drugs such as heroin and morphine that have medical uses but are known to be very potent and dangerous; they are subject to tight controls in their legal distribution. As can be seen from the fact that heroin in Schedule II is a Class A drug but cannabis in Schedule I has been either a Class B or C drug during the last decade, the two scales are distinct. Note that in both the Schedule and Class scales no weight is given to the pleasure derived from the substance; only therapeutic utility can be considered a benefit.
The Home Secretary is empowered to seek advice from the Advisory Council on the Misuse of Drugs (ACMD) before making a decision about whether and how to classify/schedule a new drug. The ACMD is an independent body of professionals who do not work for the national government. In contrast to many other European nations (e.g. the Netherlands) the UK does not have specific emergency procedures (EMCDDA, 2009b). In recent instances the time from the Home Secretary’s request to delivery of an advisory letter seems to be 3 to 6 months. The Home Office recently announced plans for emergency scheduling of “legal highs,” although it is unlikely to come into effect until late 2011.
The United States of America
The United States has a highly structured process for the scheduling of drugs. As described in the scheduling decision for BZP: “In accordance with 21 USC. 811(b) of the CSA [Controlled Substances Act], DEA has gathered and reviewed the available information regarding the pharmacology, chemistry, trafficking, actual abuse, pattern of abuse, and the relative potential for abuse of 2C-T-7, BZP, and TFMPP.” 12 There is a three-part test for Schedule I classification:
“(1) The drug or other substance has a high potential for abuse.
(2) The drug or other substance has no currently accepted medical use in treatment in the United States.
(3) There is a lack of accepted safety for use of the drug or other substance under medical supervision”
The US does have an emergency procedure. The Drug Enforcement Administration (DEA) can issue an Interim ruling scheduling a drug for 12 months (with a potential for 6 months extension). At the end of the period the DEA Administrator can only issue a final scheduling decision if the Secretary of HHS has completed an evaluation and recommends scheduling.
Note that there is no reference to harms associated with the distribution, as in the European Union Directive. The Drug Enforcement Administration, primarily a law enforcement agency, is in charge of the process but can consider only health-related consequences. However it is required to consult with other relevant expert agencies in the federal government. It must also consider information provided by any party, public or private, once the decision process begins, as is true of regulatory rule making in the United States generally.
Central to the proceedings in the US has been the Federal Analog Act, enacted in 1988, after a previous legislative effort had been struck down by the courts. Until then the DEA had to promulgate a ruling for each chemical separately. With the American regulatory and legal system’s extreme deference to due process, this took many months, sometimes years; Kay (2002) describes the four-year process needed to schedule Ecstasy. Instead, the 1988 Act automatically prohibits a chemical if it is "substantially similar in structure" to an already-prohibited drug, and has a "substantially similar chemical effect" or is "represented to have such an effect." Only 70 prosecutions have been brought under the Analog Act because its vagueness prevents prosecutors from being certain that it applies. In every case the courts have found the litigated substance to be an analogue, indicating considerable deference to the government’s interpretation of the law.
Though it is difficult to measure the phenomenon across nations in a consistent fashion, legal highs appears to be less of a problem in the US than in Western Europe. It may be that the Federal Analog Act is an important deterrent factor. Its vagueness, which can hamper prosecutors, may amplify the deterrent effect; underground distributors are also unsure whether they would be able to avoid being convicted under the Act. However, one should also note the relative severity of sentences in the United States; ten- to twenty-year federal sentences for drug supply convictions are common enough to give pause to a chemist with reasonable earnings opportunities in legal activities.
3. THE EXPERIENCE WITH FOUR NEW DRUGS
There have been surprisingly few instances in which a new psychoactive substance, not covered by existing regulations or laws, has become a significant problem. Only four have received much attention in the United Kingdom so far, though the threat is continuous13:
BZP (1-BENZYLPIPERAZINE)
This drug, briefly available in Hungary as a registered anti-depressant14, came into the recreational market as a means of ending dependence on methamphetamine (Sheridan et al., 2007). It became a widely used drug in New Zealand in the period 2004-2008, after which time the New Zealand government prohibited it. This is probably the richest and best-documented case of a government struggling with an array of choices for regulating a new drug that was popular but whose dangers were not yet well understood.15
The substance first appeared in “party pills” in 2000. The government’s initial regulatory efforts were not until 2005, when it was placed in a new schedule within the Misuse of Drugs Act as a “Restricted Substance”. This scheduling prohibited sale to anyone under 18 and prohibited various promotional activities, which had previously been widespread; otherwise the substance was unregulated (Sheridan and Butler, 2010). As reported by the New Zealand Law Commission’s (2010) review of drug policy, “BZP was the fourth most widely used drug in 2007/08. 5.6% of respondents had used BZP in the previous 12 months, while 13.5% had used BZP at some point in their lives.”
There was an intensive review of the regulatory options by the New Zealand Expert Advisory Commission on Drugs (EACD)16 in 2006 and 2007 (EACD, 2006 & 2007). The EACD concluded that the risks to users from BZP were modest; the variability of potency of preparations being sold as BZP (without formal regulation) was amongst the most important sources of risk. Acute problems were often the result of combining BZP with alcohol or other drugs. The public health risks were also assessed as modest and there was emphasis on the dangers to the population resulting from its ambiguous legal status. The population saw it as legal, whereas it was merely not prohibited. In fact the products were not subject to formal regulation, only voluntary guidelines from an industry association which were not thought to be effective. The Expert Advisory Committee concluded that, “While it is the EACD’s view that the research has now demonstrated that BZP does pose a moderate risk of harm, newer substances may be shown to pose a low risk of harm but still be worthy of restrictions. The Committee’s view is that the implementation of restrictions should place the burden of proof on the person supplying the substance to demonstrate the safety of a new psychoactive substance (EACD, 2007; p.7). In April 2008 the New Zealand government moved to schedule the drug as Class 1 under the Drugs Misuse Act of 1975; this amounted effectively to full prohibition. There was a six-month transition period in which purchase, possession and use were not yet prohibited.
The drug, whose reported adverse effects include psychosis, renal toxicity, and seizures (Freye, 2009), has also been prohibited in a number of EU member states, Australia and the United States. The US action was much earlier than those of other nations. Already in 2002 an emergency scheduling had taken place. It reflected three deaths that had been identified as involving the simultaneous use of BZP and MDMA. Between 1996, when the drug had first appeared, and 2002, seizures had taken place in 21 states and the District of Columbia. The underlying science was slender and not well interpreted by DEA, which relied on two studies from the 1970s comparing BZP to amphetamines.
The EMCDDA and Europol committee report on how the European Commission should regulate the drug was explicit about the limited evidence available.
“There is an absence of standard safety pharmacology and toxicology data. Only a few direct studies have been made on the physiological properties of BZP in humans, and nothing has been published on the effects of BZP on specific organ systems. Much of the available information derives from indirect sources, either from studies of Trelibet®, from self-reports of users on Internet sites, from clinical observation of intoxicated patients or from post-mortem material. Many of these latter ‘case reports’ involve polydrug use and therefore suffer from problems of interpretation. Many BZP tablets and capsules also contain TFMPP (1-(3-trifluoromethylphenyl) piperazine). Furthermore, surveys in New Zealand have shown that most users consume BZP with alcohol as well as other psychoactive substances.” (EMCDDA, 2007; p.4)17
The committee recommendations were thus appropriately very cautious. There was no evidence that the drug was safe for human consumption but the risks seemed modest. The committee did not firmly recommend control. Instead it equivocated, emphasizing in its final sentence the weak evidence base: “Many of the questions posed by the lack of evidence on the health and social risks of BZP could be answered through relatively simple and inexpensive research. A strong conclusion of the Committee was that further studies are needed, especially in respect to potential neurotoxicity and social consequences.” (p.9) The appropriate European body (European Council) in 2008 recommended that Member States put BZP on the list of controlled substances.
The Home Office asked the ACMD to review BZP in 2008, after the EMCDDA report was published. Already in 2007 the Medicines and Health Care Products Regulatory Agency (MHRA) had issued a press statement warning that sellers of BZP were potentially subject to prosecution18. The ACMD’s task was simply to provide advice on the implementation of the European Council requirement that BZP be placed among controlled substances. The brief ACMD report added little to the existing literature and focused on whether only BZP should be included, or whether it should be a broader class; it decided on the latter19.
SPICE
Spice is a set of herbs with some synthetic cannabinoids added. The regulatory issues have been analyzed by the EU system (Griffiths et al., 2010; EMCDDA, 2009). Spice is poorly defined. Its active ingredient could be any one of a large number of cannabinoids that are not regulated and about whose harms little is known. Further complicating matters, it often does not contain the materials that are identified on the package.
It has been very much an Internet phenomenon, where it is advertized for purposes other than consumption (e.g. incense). “The United Kingdom noted that a Google search produced over 11 million hits for Spice Gold (higher than most of the commonly used illicit drugs)” (EMCDDA, 2009, p.14).
It was first identified officially in Sweden in 2007, though it had been available at least since 2004. There was an EMCDDA expert meeting early 2009, which surveyed Focal Points20 as to whether there was any evidence of Spice being available in their countries. Among other indicators, the survey considered web sites advertizing either in the country or aimed at the country. The drug was not available on sites in countries that had prohibited the drug. Where it was available the price per dose was comparable to the price of cannabis per joint, approximately 3-4 euros.
Though it was available from almost half of a sample of online shops that were surveyed, the reports from Member States showed a great deal of variation in availability. For example, “the French Early Warning System suggested that the presumably target population for these drugs (mainly party goers) showed little interest in these types of products.” (EMCDDA, 2009, p.13) No mention was made of any organized crime involvement.
The EMCDDA/Europol expert group was unable to reach a firm conclusion about any aspect of the substance: its harms, popularity or sources21. Indeed, when written up for journal publication (Griffiths et al., 2010), the drug was offered as a case study in the problems presented by globalization and market innovation with an emphasis on complexity and uncertainty.
The Advisory Council on the Misuse of Drugs was willing to go further, though it did not conceal the highly speculative basis of its recommendation that the drug be classified under the Misuse of Drugs Act in the same schedule as cannabis.
“Our report explains that the detailed pharmacology of these synthetic Compounds [in Spice] is, as yet, unknown. There are also a large number of potential cannabinoids that could be synthesised. However, some inferences can be made based on the chemistry of the drugs identified to date and it is very likely that these synthetic cannabinoids will produce harmful effects similar to those associated with THC. Indeed, our report notes that the substances containing the synthetic cannabinoids have the potential to be
more harmful than cannabis due to their method of manufacture and that the compounds present and their quantity (and hence potency) is unknown to the user.
After consideration of the available evidence, the ACMD concludes that with respect to the classification of substances under the Misuse of Drugs Act, the harms of the synthetic cannabinoids are broadly commensurate with those of cannabis and that they should be classified accordingly.” (ACMD, 2009; pp.2-3)
In the United States, Spice has not received much attention and, as noted before, did not appear in the DAWN system of information from Emergency Departments and Medical Examiners as recently as 2010. For example, in discussing major components of Spice, DEA refers to its occurrence in European recreational markets and not in the United States.22 The same document also mentions just an occasional seizure of a drug with that name.
Nonetheless, on November 24, 2010, the DEA announced that it proposed “using its emergency scheduling authority to temporarily control five chemicals (JWH-018, JWH-073, JWH-200, CP-47,497, and cannabicyclohexanol) used to make ‘fake pot’ products…. Since 2009, DEA has received an increasing number of reports from poison centers, hospitals and law enforcement regarding these products. Fifteen states have already taken action to control one or more of these chemicals” (DEA 2010). No information beyond the press release was provided.23
MEPHEDRONE
Mephedrone (4-methylmethcathinone) is a substituted cathinone (an ingredient of khat) commonly known as Miao/Meow or TopCat. It is the most recent legal high to attract attention, particularly in the United Kingdom, which accounts for 88% of European seizures of the drug (EMCDDA, 2010). Measham et al. (2010) argue that it has emerged in part as the consequence of problems in the Ecstasy market, where consumers have found it difficult to obtain product of consistent quality.
Mephedrone provides Ecstasy-like effects, though also some stimulant effects more characteristic of amphetamine.
Once again knowledge of the adverse effects of the substance is slight, though in contrast to Spice it is well defined. The 2010 report by the EMCDDA states: “There are no formal pharmacokinetic and pharmacodynamic studies on mephedrone. There are no published formal studies assessing the psychological or behavioural effects of mephedrone in humans. In addition, there are no animal studies on which to base an extrapolation of potential effects. Therefore psychological and behavioural effects related to mephedrone use are based on users’ reports and clinical reports of acute mephedrone toxicity.” (p.12) The user reports used in the analysis derived from an on-line survey of subscribers to MixMag, a dance music magazine. A wide variety of negative effects were reported and some by a large fraction of the users.
A survey of EU Member States found that only the UK and the Netherlands had made substantial seizures; the nine others with any seizures reported totals of between 2 and 325 grams. Eight other member states reported no seizures of mephedrone.
The United States has yet to see any substantial sign of mephedrone, though in January 2011 there was reporting of a problem emerging in Mississippi (Byrd, 2011). One state, North Dakota, has scheduled the substance but an interview with an executive of the state agency responsible for the decision suggests just how little evidence is needed for such decisions at the state level. What follows are notes from Alexa Briscoe:
“The Bismarck Tribune ran four stories on mephedrone around the time it was placed under Schedule I within the state, via an emergency ruling24.
The agenda for the emergency scheduling meeting states: “The purpose of this rule is to schedule substances which have an actual or relative potential for abuse; and which bear risk to the public health by unknown individuals using them by inhaling the smoke, vapors or by ingesting or injecting the substances”; however, I have been unable to find any sort of study conducted by North Dakota to demonstrate such potential for abuse and health risks, or even any review of existing studies or cases.
In response to an email, an official of the North Dakota Pharmacy Board said: ‘We had a couple of teenage girls in the hospital here after injecting the "bath salts" intravenously, presumed to contain mephedrone. The news reports and general research into the drugs chemistry and effects were enough for the Board and the Attorney General. When the lab report came back it was actually 3,4-methylenedioxypyrovalerone (MDPV) so we scheduled that one, as well.
We have not heard any good reasons why we should change the action, so they will stand and the legislature will get a chance to review the action in our 2011 session.
If we had truth in labeling for Bath Salts, K-2 and Spice products, maybe protection of the public would not require the scheduling. It makes it pretty tough for the emergency room physicians to treat someone when they have no idea what the patient has used.’”
NAPHYRONE/NRG-1
Naphyrone is a high-potency cathinone; harms closely equate with those of mephedrone, but the standard dose of naphyrone is one-tenth as large. It is sold in the UK largely through the internet. The ACMD (2010) states that a majority of samples described as containing naphyrone actually contain mephedrone and other related compounds25. Indeed the ACMD speculates that this may be the consequence of mephedrone distributors dumping their produce under a nonprohibited label, now that mephedrone itself is listed under the Misuse of Drugs Act.
Naphyrone has considerable abuse potential. It is a triple-uptake inhibitor like cocaine (with ten times the potency) rather than a single-uptake inhibitor like damphetamine and MDMA. The lack of transparency regarding content could easily
lead to overdoses given the relative strength of naphyrone26.
The ACMD noted that “there have been no confirmed cases of acute toxicity associated with the use of naphyrone.” (pp.11-12) Further: “The ACMD is not aware of any relationship between naphyrone and anti-social behaviour or acquisitive crime.” (p.13) In recommending classifying it as a Class B drug, the ACMD emphasized that (1) drugs marketed under this name often contained other drugs (2) the differences in dose size between naphyrone and the drugs often sold under that name were large and (3) the effective dose size of naphyrone was so small (25 mg.) that many users were likely to take dangerously high doses.
David Nutt, former chair of the AMCD who was fired for his outspoken views, has been highly critical of the decision. He notes the absence of evidence of either harm or popularity and argues that the decision can have adverse consequences for medical research, since it will inhibit exploration of a family of chemicals that might have value in the treatment of addiction (Nutt, 2010).
The drug is not regulated in the United States at present.
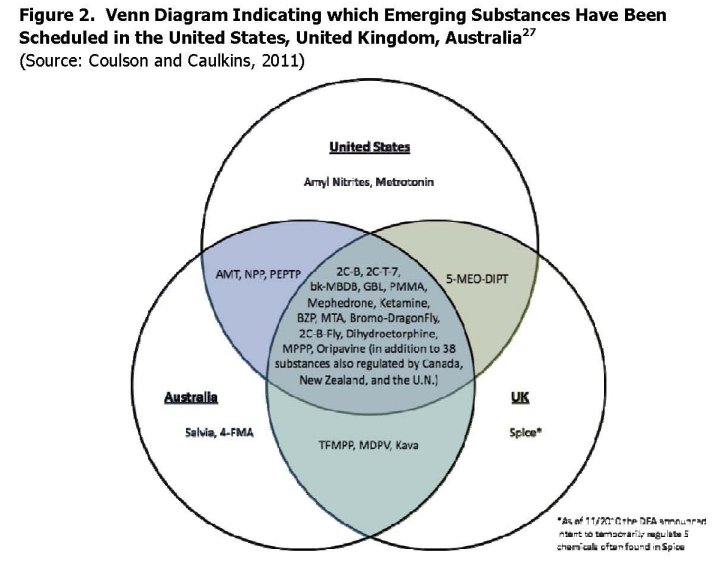
Figure 2, taken from Coulson and Caulkins (2011) shows how emerging substances have been scheduled in the UK, US and Australia. As they note, there is a great deal of overlap across the three countries. Of the 38 substances on the list, 26 (two thirds) are scheduled in all three jurisdictions; only 5 are scheduled in just one jurisdiction. There are in addition 38 substances that are scheduled not only in these three countries but also by Canada, New Zealand and the United Nations.
4. LEARNING FROM OTHER REGULATORY FRAMEWORKS
As noted in the Introduction, there are quite different models of regulatory decision making that might be considered appropriate for making decisions about these new drugs. We consider two alternatives here: the system used for alcohol and tobacco
in many nations and that used for food supplements in the United States
ALCOHOL AND TOBACCO REGULATION
Given the continuing interest in making comparisons between the harmfulness of the two principal legal drugs, alcohol and tobacco, and of those that are banned under the Drug Misuse Act, it has been suggested that the alcohol and tobacco regulatory systems could provide a useful model for comparison. There is an international Framework Convention on Tobacco Control but that is far less intrusive on individual nations’ policies than the conventions for psychoactive drugs. There is no counterpart for international alcohol control. Western countries have developed elaborate and highly intrusive regimes for these two products, governing both the nature of what can be sold and how it can be promoted. Though many observers argue that the existing regulatory regimes are insufficiently stringent (see Cook, 2007 for an assessment of alcohol control), in many countries they have been tightened in recent years. In particular, restrictions on promotion and taxation of cigarettes are vastly tighter than were a generation ago.
Yet as schemes for the assessment of new products, there seems little to be learned from either substance. New alcohol or tobacco products may attract regulatory attention if they represent an exaggeration of existing forms, as for example when attention was drawn in the U.S. to drinks that contained caffeine as well alcohol; see the FDA notice banning certain products28. However regulation has not had to deal systematically with the issue of distinctive new substances.
REGULATING UNDER FOODS OR RELATED LEGISLATION
Another alternative approach to regulating these new substances that make no therapeutic claim is to treat them not as drugs, for which the government has to make safety and efficacy determination before letting them on the market, but instead like other products where there is a presumption of safety which can be challenged when the government has gathered data indicating dangers. This is a common approach to the regulation of food in Western countries.
The experience with weight loss products in the United States offers a sobering lesson. These products provide a particularly strong analogy because the producers provide a continuing string of apparently new products, making claims about them which are difficult to validate.
The weight loss products are regulated by the FDA (Food and Drug Administration) but as foods rather than drugs; see GAO (2002) for a full description of the system. The presumption is that foods are safe until proven otherwise; the burden of proof is on the government to show that a product is unsafe rather than on the manufacturer to show that it is safe. If a food or weight loss product is found to be unsafe or dangerous, then the FDA can require that the product be pulled from the market. The Federal Trade Commission (FTC) can, and does, regulate the advertizing for these products. Most importantly, the producers may not claim the product cures any disease or illness.
The market for these weight loss products in the United States is estimated to be about $2 billion per annum (Cawley, 2011). Most of the products marketed are at best ineffective; some are harmful, a few extremely so. The advertizing ranges from the inaccurate (“lose weight while you sleep”) to the ludicrous: “7 weeks ago I weighed 268 lbs, now I am down to just 148 lbs! During this time I didn’t change my eating habits at all: “This latter claim is equivalent to a daily deficit of over 8,000 calories. (FTC, 2002)
The fact that these products are distributed in a legal market provides false reassurance about government regulation. Approximately half of American believe that the weight loss products are approved for safety and efficacy before they can be sold to the public (Pillitteri et al., 2008). The same ignorance held for young physicians: over a third of physicians in residency programs believed that these products needed to be approved by the FDA before they could be marketed. The FDA has difficulty obtaining information about adverse events involving these products (GAO, 2002) since the producers do not have an obligation similar to that of pharmaceutical manufacturers to monitor post-market experience. The GAO (2002) report described the problem in detail.
“It is more difficult to study the safety and efficacy of multiple-ingredient products because each product may have a different combination of ingredients, meaning that each individual product would need to be studied. Further, the amounts of ingredients in a product may be unknown if the product contains a “proprietary blend” of various ingredients. Proprietary blends must list ingredients but are not required to specify the amount of any individual ingredient. Finally, it is harder to identify patterns in the adverse events associated with multiple-ingredient products and attribute the events to either an individual ingredient or a combination of ingredients. A study found that in 95 percent of the adverse events reported to FDA for products containing chromium, the products also contained as many as 11 additional ingredients, any of which may have been responsible for the adverse event. It is also possible that it is the interaction of these ingredients that is responsible for the adverse events.” (p.10)
Efforts to restrict false advertizing are weakened by the lack of certainty as to the composition of any product. The FDA and FTC regularly file suits against the manufacturers on safety grounds (the FDA) or false advertizing (the FTC). State and local authorities also regularly file suits and occasionally obtain substantial awards against the manufacturers. Nonetheless the problem continues, reflecting the fly-bynight nature of many of the manufacturers; they move from one banned substance to another, with a different name both for the product and the manufacturer and many of the same ingredients.
There are many ways in which the regulation of these weight loss products and their advertizing could be strengthened. For example, the producers could be required to conduct post-market monitoring and to list all ingredients. Nonetheless, there is little in the American experience with weight loss drugs to recommend this regulatory approach for new psychoactive drugs. While it would prevent or delay the adverse consequences of prohibition for drugs that turn out not to be harmful, it allows those that truly are dangerous to linger in the market, with the apparent endorsement of the government, for a long period.
5. REGULATORY OPTIONS: GENERAL CONSIDERATIONS
What are the options for a government facing a new substance, about which not a lot is known but for which there are some indications both of potential harms to users and a growing popularity, whether for recreational or self-medication purposes? Table 1 and the four cases briefly summarized above point to the dilemmas of making decisions with limited information for which one outcome (a dangerous substance has not been prohibited) is far more salient than others. They can be seen to illustrate the application of the precautionary principle, a major advance in thinking about the regulation of health and environmental hazards.
THE PRECAUTIONARY PRINCIPLE
The problem of choosing regulatory options for new psychoactive substances is, analytically, not unique. It is simply another instance of the government being required to make regulatory decisions under uncertainty. For this, in recent years, great attention has been given to the “precautionary principle”. Though its application in environmental policy decisions has been given much more emphasis, it is useful to examine its applicability to drug regulatory decisions.
The definition of the principle that is most widely quoted is from the 1992 Rio Declaration, which states that: “where there are threats of serious or irreversible damage, lack of full scientific evidence shall not be used as reason for postponing cost-effective measures to prevent environmental Degradation”. It is, as stated there, a very modern principle. Scientific knowledge is seen as an essential element for making policy decisions, in particular setting regulations. Very often, indeed almost invariably, the scientific base for a decision is vulnerable to criticism, as can be seen by the frequency with which U.S. courts sustain challenges to regulatory decisions on the basis of weak evidence.
In what circumstances can the precautionary principle be invoked? The European Commission, implementing the Council’s adoption of the principle in its Resolution of April 13, 1999, states:
The precautionary principle may be invoked where urgent measures are needed in the face of a possible danger to human, animal or plant health, or to protect the environment where scientific data do not permit a complete evaluation of the risk. It may not be used as a pretext for protectionist measures. This principle is applied mainly where there is a danger to public health. For example, it may be used to stop distribution or order withdrawal from the market of products likely to constitute a health hazard.29
The phrasing could not be more appropriate to decision-making involving new drugs, about which the science is indeed uncertain and which pose a potential risk to public health.
The precautionary principle is controversial even in its intended setting. Cameron (2006) in an analysis of its applicability to environmental regulation suggests that it is a very narrow application of general risk management. It is, in the simple form, only applicable when all parties are risk averse and the dangers large and irreversible. Implicitly, Cameron is arguing that it privileges just one element of the decision maker’s considerations.
APPLYING THE PRECAUTIONARY PRINCIPLE TO DRUGS
In political terms, the principle is seen as liberal, a way of defending active environmental regulation against a major source of attacks by corporations. However, in the context of drug-specific regulation, it is a conservative rule. There is, with all the psychoactive substances considered so far, some evidence of dangers to users, if not to others. The principle affirms that the state has an affirmative duty to protect individuals from such hazards; hence it appears to favour scheduling. Classifying a drug as falling under the Misuse of Drugs Act is tantamount to prohibiting its commerce, since at the time of the decision, it will not have been through sufficient testing to avoid being put into Schedule 1, “no recognized medical use”. Indeed, these new substances are not presented as therapeutic drugs.
However the complication here is that giving deference to those harms ignores the harms that arise from the decision to prohibit. The ubiquitous David Nutt has presented an articulate attack on the application of the precautionary principle in two recent writings, briefly in Nutt (2010) and in more detail in a blog entry dated 23 June, 201030. He identifies eight specific harms from application of the precautionary principle. For example he points out that some of the substances banned might turn out to be substitutes for alcohol that cause less harm than alcohol to the user and to others. Separately he points out that the principle inhibits the development of substances explicitly intended to substitute for alcohol since they would be subject to much tougher scrutiny than that drug. His case is powerful.
There is general recognition that the decision to prohibit a substance may be harm enhancing, at least in the gross sense; see for example New Zealand Law Commission (2010). Prohibition means that those involved in the production and distribution are now at risk of arrest and various penalties. That will inter alia affect who stays in the business. Some entrepreneurs who are willing to participate in a marginal activity without clear legal status will be unwilling to undertake a clearly illegal activity. That will leave a more risk-seeking and probably more violent group of entrepreneurs. It will also affect the character of the organizations involved. Organized crime (referring to organizations that have more general criminal skills, including a capacity to corrupt and make credible threats of violence) may now become an important element of the production and/or distribution.
However it is worth noting that large segments of the distribution and production of drugs are minimally affected by organized crime. For example, much of the marijuana market is essentially social rather than criminal in its orientation, with friends providing each other; see Caulkins and Pacula (2006) for an analysis of survey data on the sources of marijuana for users in the United States. The Ecstasy market also has little connection to organized crime. For Europe, Paoli and Reuter (2008) observe considerable differences across drugs in various aspects of their distribution, including the involvement of broader criminal groups.
Even if organized criminals remain uninvolved, there are a variety of other harms following from prohibition. Prohibition eliminates the possibility of providing quality control and assurance. Harms to individual users, on average, will rise as a consequence. Another effect, which weighs heavily on regulatory agencies, is that it complicates the task of gathering data on the substance that would allow for a more informed decision, since there are no manufacturers with an incentive to collect and analyze such data; moreover it is believed that prohibition reduces the willingness of respondents in studies to disclose use.
In making these decisions there is a pro forma argument for considering whether the new substance is substituting for a more dangerous prohibited one. The New Zealand data on BZP, whose use became very widespread before it was prohibited, provides the best evidence on this. As summarized by the NZ Drug Foundation, there is some evidence that “legal” BZP was less a gateway to other, harder drugs than it was a cheaper and more accessible alternative to them. The New Zealand Expert Advisory Committee on Drugs (EACD, 2006) noted that “Substitution of illicit drugs with piperazines is occurring, mostly amongst users who are afraid of the damage to their lives that a conviction would bring and who also wish to normalise the transaction required to purchase their choice of recreational substance.” (p.8) If that were true, then a comprehensive analysis of prohibiting BZP would have to include the potential for increase or decrease in the use of other, more dangerous drugs.
This would require a highly speculative line of analysis. The BZP data in New Zealand was exceptionally rich for a new substance precisely because the government had left it on the market for so long and it had become so popular; thus the potential for substitution was better understood. But as Sheridan and Butler (2010) report, the apparent legality also encouraged users to take more than recommended doses; thus even if the drug was substituting for other more dangerous ones, it may have been taken in dose levels that reduced the health advantages implied. More typically the government is required to make a decision (which could include postponing a decision) at a time when little data on drug substitution is available. The time it takes to gather convincing epidemiologic data is certainly years, perhaps even many years. It would be a brave scientific committee indeed that would be willing to venture, on the basis of the limited data available, that the potential for substitution was high enough to make permitting of the drug, or at least delay in prohibiting, a sound decision.31
A major concern expressed in the few major decisions to date has been the advertizing of the new substance, in particular on the internet. This has allowed new substances to reach groups rich in potential consumers much more rapidly than in previous eras. One strategic option is to slow the spread of the drug while gathering more information by regulating it under another regulatory regime, such as the one governing medicines, in which in the UK and most other Western countries, direct advertizing to users/patients can be prohibited.32
However, as noted above, any kind of implicit legalization has unintended consequences. A risk factor for users of illicit drugs is uncertainty about the contents of what they acquire. The regulatory alternatives can require that packaging provide a full list of ingredients, thus reducing the risk of an unanticipated reaction.
However, that reduced uncertainty may encourage taking larger quantities, as noted above in the discussion of BZP in New Zealand33 This argument runs the risk of cynicism. The lack of quality assurance can also of course lead to deaths. The above statements merely recognize that there are complex effects, some in the other direction.
The problem has some symmetry with that facing drug regulatory agencies as to what criteria they should use in deciding whether to allow a therapeutic drug into the marketplace (Eichler et al., 2008). The regulatory agency may be criticized for withholding the benefits for too long if the standards for assessing safety, efficacy and benefits are too stringent. Stringency also raises the costs of bringing a drug to market and may thus discourage innovation. Conversely, laxer standards will occasionally lead to marketing of drugs that are unsafe, ineffective or more costly than beneficial. The trade-off is clear and there is no generalized right answer.
The uncertainties surrounding assessment of risks related to medicines is worth emphasizing. Consider Avandia, a widely prescribed anti-diabetes drug that aimed at reducing the risk of heart problems. After 11 years on the market, evidence has accumulated that for some classes of patient, far from reducing the risks of cardiovascular events such as heart attack and stroke, the drug in fact raised them. In the fall of 2010 the European Medicines Agency recommended that Avandia be withdrawn from the market and the US Food and Drug Administration imposed new restrictions. “Avandia will be available to new patients only if they are unable to achieve glucose control on other medications and are unable to take Actos (pioglitazone), the only other drug in the class.” The agency also required additional
review of data from trials that had been ordered three years earlier by the European Medicines Agency (EMA), raising questions of integrity in the conduct of the trial. The EMA introduced new restrictions on the distribution of the drug.34 The fact that these decisions, even though drawing on large and elaborate clinical trials, are so often later reversed or at least viewed as questionable, indicates the difficulty of assessing the effects of drugs on humans.
There is active discussion of developing new regulatory options as a way of dealing with the dilemma for new therapeutic drugs. One such option is conditional or accelerated approval. Where there is great need for the drug, the regulatory agency may accept a higher risk level for early approval, at the same time requiring that the manufacturer continue to collect data that would allow, by a specified date, a full review. Another option is labeled “staggered approval”, whereby on the basis of limited data the agency authorizes use of the drug to a narrowly defined patient group with the greatest need. As more data becomes available, the authorization can be expanded to more patient groups, defined by therapeutic need. Now compare the decision and setting for a psychoactive substance that has just recently emerged clandestinely from the thicket of chemists and distributors who operate at the margins of the legal psychoactive drug industry. There are parallels in the structure of the decision and the consequences of errors. Prohibiting the drug hastily can create the problems associated with illegal markets unnecessarily as well as make it impossible to gather data for a more informed decision. On the other hand prohibiting it too late may allow some drugs that are addictive and/or dangerous to the user to become widely used.
The differences in the decision processes for therapeutic drugs and emerging psychoactive substances are more striking. An important distinction is the setting of the decisions. There is a well-financed and effective lobby for both sides of the regulatory decision debate for pharmaceuticals developed by pharmaceutical manufacturer. The manufacturers are often very large corporations, anxious to recoup investments that may run to the hundreds of millions of dollars. The consumer side is often represented by well organized NGOs, provided with information from other government agencies that gather relevant data. Egregious errors are likely to generate effective protest by the injured party.
In contrast those who wish to prevent new backyard psychoactive drugs from being listed as prohibited drugs are marginal and poorly organized. Because the drug is new, they will not be able to enlist a large number of users to protest denying access to what they might see as a harmless (or less harmful) pleasure. Egregious errors on behalf of prohibition are unlikely to generate effective protest.
The decision is weighted against approval in another sense, the most fundamental of all. No weight is given to the benefits of the substance to the user. At best the regulator might take into account that a substance is “less bad” than the alternatives. Thus the only benefits from not prohibiting the drug that are considered are precisely those, the benefits of not prohibiting. There is nothing within the process that allows the fact that, if the drug was allowed to enter the market, perhaps many users would gain pleasure without adverse consequences, to be taken into account.
6. WHAT SHOULD BE CONSIDERED IN MAKING DECISIONS ABOUT PROHIBITION AND SCHEDULING?
Caulkins and Coulson (2011) make a very important point when they note that there have been no major disasters (large numbers of deaths or serious injuries/infections on the one hand; large and violent illegal markets on the other) associated with new substances in recent years. The system may be too cautious but it has apparently not made many errors on the other side, allowing dangerous drugs to be legally distributed. The problem nonetheless remains potentially a serious one for developed nations. The growth of sophisticated chemical labs, legal and clandestine, in developing countries with minimal regulatory surveillance makes the future threat look particularly troubling. The hazards are amplified by the discovery of new uses of a growing array of existing substances.
There is a history of critiques of the systems applied to regulating the distribution of psychoactive substances; for recent commentary see for example Kalant (2010), Nutt, King, Saulsbury and Blakemore. (2007) and Nutt, King and Phillips (2010) . The most common criticism is that the results of the system lack rationality; drugs that are very dangerous (most notably alcohol and cigarettes) are hardly regulated at all, whereas substances with fairly modest harms to individuals and societies are prohibited (Nutt, 2009). The decision by Prime Minister Gordon Brown in 2008 to schedule cannabis as a Class B rather than a Class C substance, before hearing from the Advisory Council on the Misuse of Drugs, only increased cynicism in Britain that the system reified social and political attitudes rather than resulting from scientific analysis. Twenty years earlier, a similar decision by the Administrator of DEA on keeping cannabis as Schedule I, overturning a well documented positive finding by an administrative law judge, had generated similar cynicism in the USA.35
David Nutt and others have attempted to define the inherent risk of psychoactive substances as the basis for making regulatory decisions, including prohibition (Nutt et al. 2007). This however is only one factor that governments need to take into account, particularly with respect to prohibition. For example, the likely adverse consequences of creating an illegal market is one possibly important characteristic omitted from the analysis (Nutt et al., 2007). Some chemical entities, which can only be acquired by diversion from large scale and sophisticated pharmaceutical production system (e.g. methaqualone) may present much less of an enforcement problem than others that can be relatively easily be manufactured by low skilled workers in small batches, occasionally with substantial environmental hazards36. The size of the existing user base is another factor that can affect the choice between prohibition and regulation. Turning a large number of otherwise law abiding citizens, who are habitual users, into criminal offenders is not a decision to be taken lightly.
The Advisory Council on the Misuse of Drugs (ACMD, 2010a) recently advanced a new and more sophisticated criterion using Multi-Criteria Decision Analysis (MCDA), a well known tool for assisting decision makers in a variety of regulatory contexts. It involves enumerating all the relevant harms and weighting them so as to take account of the perceived importance of each one. The test model that the ACMD proposed, with many appropriate caveats, involved 16 different harms, nine borne by the user (e.g. increased risk of morbidity and of mortality) and seven by the rest of society (e.g. acquisitive crime or family breakdown). See Nutt et al. (2010) for another application of this analytic approach.
There are two limitations to the MCDA approach that are relevant for the legal highs issue. The more fundamental is that the harms are assumed to be intrinsic to the drug rather than the result of the drug and its regulation. That is clearly false. For example, the mortality associated with heroin use is much lower if it is purchased in known quantity of specified purity from a pharmacy for injection with a sterile needle rather than purchased in a clandestine transaction with unknown adulterants to be injected with a used needle. Obviously acquisitive crime is influenced by price, which will be much higher if the drug is prohibited.
The second limitation, paucity of data, applies particularly to the legal highs. Though the Advisory Council notes that information is likely to be incomplete, it does not confront the utility of the tool when there is almost no information. For the new substances that constitute the category of legal highs, a fair description is that the tool requires almost complete guesswork for most entries.37
Box A provides an expanded list of candidate harms that might be considered in making decisions about whether to allow a substance on the market. A scan of that list suggests just how few of them involve information that is likely to be available at the time of regulatory decisions.

7. CONCLUDING COMMENTS
Among the few analysts that have written seriously on this issue of regulating new psychoactive drugs intended for recreational use, there is considerable unease with the existing system for making decisions about newly emerging psychoactive substances38; see Caulkins and Coulson (2011) on these critiques. Many countries require that each substance be subject to its own extensive review, which is cumbersome when the flow of new substances with potential markets is threatening to turn into a flood. Use of analog or generic systems rather than individual drug listings and introduction of accelerated procedures can reduce the burdensomeness and delay in making decisions but only in terms of increasing the ease of prohibition.
However I believe that there are more fundamental concerns that the system is biased toward prohibition. This paper argues that the bias toward prohibition and scheduling is almost impossible to avoid. The adverse consequences of mistakenly refraining from prohibiting what may turn out to be a dangerous drug are massive both for the individual decision maker and for the political party in power at the time. On the other hand the gains from correctly allowing a new psychoactive substance to enter into the market, with appropriate regulatory controls, are modest and not very salient for the decision maker or the government. A Type II error (allowing what should have been prohibited) has much greater consequences than avoiding a Type I error (prohibiting what should have been allowed). That will be true even with a broader array of legal options. Once the decision not to prohibit is explicit, the decision maker faces a risk of significant public retribution.
There has also been suggestion of moving to a completely different regulatory system, as suggested in the Introduction, one in which the government is not required to make a decision on prohibition as early as currently under the Misuse of Drugs Act or its equivalent in other nations. I believe that even under a system such as that used for foods or medicines there will be the same bias, particularly since “not prohibited” is so easily misinterpretered as “permitted by the government”.
The major task then is to develop a means to override this bias and to ensure a more balanced set of regulatory decisions. That surely requires a change in attitudes of the public toward these substances. Since the end of World War II, if not even earlier, Western societies have become increasingly tolerant of different ways of seeking pleasure, notwithstanding the occasional shift toward tighter controls, as has occurred in roughly the last decade in much of Western Europe. However this refers to phenomena that are well beyond the scope of any design of drug-specific regulation and to periods of time that are too long to be useful. Emphasizing substitution, that these substances may be less harmful than those that are already being used, whether legally or illegally, may be a more promising strategy for persuading the public that there can be gains from allowing regulation of risky new drugs.
Finally I return to the Caulkins and Coulson (2011) observation; the system has worked well in the sense that no major problem has emerged from a decision to allow into commerce a drug that turns out to be dangerous. That is no small accomplishment. The system may deny the populace some new pleasures and expand the harms of illegal markets. These are more subtle and intangible gains but not necessarily minor; a less harmful substitute for alcohol would be worth a great deal. However, the burden of proof for creating an alternative system is a heavy one. A research effort to improve understanding of the substitution effects of new entities, as well as an examination of how prohibition of these entities creates market harms, would go a long way toward clarifying the issues.
Peter Reuter1
1 School of Public Policy and Department of Criminology, University of Maryland. Alexa Briscoe provided excellent research assistance. I have also benefited greatly from access to two unpublished papers by Jonathan Caulkins and Carolyn Coulson. Brendan Hughes was very helpful in discussing EU procedures. The final version benefited from Allison Ritter’s extremely useful review.
2 In 1999 8.1% of high school seniors reported use of LSD in the previous month; that figure was 1.6% in 2009.
3 For example, in 1996 4.0% of US high school reported use of PCP (phencyclidine) in the previous 12 months. That figure fell steadily over the next 13 years; by 2009 it had fallen by almost 60% to just 1.7%. There have been no claims of enforcement success involving this
particular drug.
4 At the end of 2009, the total number of substances reviewed since the system began in 1997 was 97.
5 DAWN collects data from a sample of hospital Emergency Departments on drug-related admissions.
6 Interestingly, Monitoring the Future asked about Salvia in just one year, 2008: 5.7% of high school seniors reported use in the previous twelve months. This made it the fourth most popular drug for that age group.
7 The 2005 Council Decision succeeded an earlier program with a narrower scope: the Joint Action on New Synthetic Drugs, that had been in operation since 1997.
8“One such group-generic definition is by reference to precise compounds which are structurally derived from a specific psychoactive substance. In the UK case of R v Couzens and Frankel in 1992, it was specified that “the term ‘structurally derived from’ does not
describe a process, but rather defines certain controlled drugs in terms of their molecular structure.” Hughes and Blidaru (2009; p.6)
9 I thank Brendan Hughes of the EMCDDA for considerable help on this matter.
10 :
11 See for example the discussion of risk assessment in British environmental regulation in Pollard (2001)
12
13For example, late in the writing of this report, the Economist (2010) reported great concern in Poland about the sudden emergence of a variety of new drugs, called “afterburners”.
14 The tangled tale of BZP’s origins is presented in EMCDDA (2007; p.3)
15 There is a relatively large descriptive literature on BZP in New Zealand. See e.g. Gee et al., 2005; Wilkins et al., 2006 and Sheridan et al., 2007
16 The Committee membership includes both medical and law enforcement personnel. The tone of the Committee reports is very much that of a medical or public health document, with careful attention to peer reviewed studies and an emphasis on evidence.
17The discussion of interaction with alcohol raises an important issue. If, despite label warnings, it is predictable that users will take the two substances simultaneously, should that figure in the risk assessment?
18 The agency stated that “There are piperazine containing medicines for human use which must be sold in pharmacies. Any other pills containing piperazine or its salts or derivatives would be classified as unlicensed as there are no safeguards in relation to the safety, quality or efficacy of the pills.” See
19 The class was defined as "1-benzylpiperazine and any compound structurally derived from 1-benzylpiperazine or 1-phenylpiperazine by substitution in the aromatic ring to any extent with alkyl, alkoxy, alkylenedioxy, halide or haloalkyl substituents, whether or not substituted at the second nitrogen atom of the piperazine ring with alkyl, benzyl, haloalkyl, or phenyl substituents." (ACMD, 2008)
20 Each Member State has a Focal Point responsible for provision of data to the EMCDDA.
21 The EMCDDA has not issued an official report under the 2005 Council Decision . A box on the first page states “This report is not an official EMCDDA publication, it is prepared to inform a specific group of recipients and is, therefore, not suitable for wider public
dissemination. The report has no legal meaning under the terms of Council Decision 2005/387/JHA.
22 CP 47,497 and homologues 2-[(1R,3S)-3-hydroxycyclohexyl]-5-(2-methyloctan-2-yl)phenol) [Purported Ingredient of "Spice"]
23 It is unclear that the ban, which was supposed to be in effect 30 days after the issue of the Notice had actually been put in place. A troll of drug activist web sites turned up a number commenting that there had been no follow-through. Whatever uncertainty existed
was clarified by March 1, 2011, when a temporary order was issued:
24 For example, 2/24/10: “Legal highs” hit Bismarck (
25 The principal source of this claim is Brandt et al. (2010) which reports on just 24 purchases from 18 websites.
26 That is an example of the importance of the social context of a drug. The harm is not inherent in naphyrone but in how it fits into the historical development of psychoactive substances.
27 38 substances that have been scheduled everywhere are not listed explicitly for sake of clarity.
28
29
30
31 A similar issue has arisen in the context of decisions about the marketing of “safer” cigarettes. A critical issue is whether these would result in more users, now relieved that the health consequences of smoking have been lowered. An Institute of Medicine panel reckoned that it would take so long to learn about the effects on incidence that it was not proper policy to allow that experiment. See Stratton, Shetty, Wallace and Bondurant (2001).
32 This is probably not an option in the United States, where the courts have been highly protective of free speech for corporations, not even allowing states to prohibit roadside advertizing of alcohol. In this setting prohibition may be the only method for limiting promotion.
33 A recent BMJ editorial by Kings College addiction researchers (Winstock et al., 2010) argued that it should be possible to provide information about dangers to users without incurring the costs of prohibition. It constitutes an optimistic read of the power of such information campaigns.
34 For a recent statement on these actions see:archive/2010/09/23/avandia-off-market-in-europe-fda-to-restrict-its-use-orders-probe-of-gskstudy.aspx
35 For the administrative law judge’s decision see A defense of the current scheduling can be found at Basis for the Recommendation for Maintaining Marijuana in Schedule I of the Controlled Substances Act, 20037–20076, Department of Health and Human Services, Volume 66, Number 75, Federal Register, 18 April 2001. Retrieved on 2007-04-28
36 Small scale manufacture of methamphetamine in the US is known to have caused considerable danger to the workers and others in their households.
37 Caulkins and Coulson (2011) examine the availability of published research at the time that US scheduling decisions were made for 9 drugs. In four cases there were fewer than 5 published articles available..
38 Obviously this discussion does not deal with new substances developed by pharmaceutical companies which will be submitted for regulator approval before being marketed.
REFERENCES
ACMD (2008) Control of 1-benzylpiperazine (BZP) and related compounds. Available at: view=Binary
ACMD (2009) Consideration of the major cannabinoid agonists. Available at:view=Binary
ACMD (2010) Consideration of the naphthylpyrovalerone analogues and related compounds. Available at: + ://www.homeoffice.gov.uk/publications/drugs/acmd1/naphyrone-report?view=Binary
ACMD (2010a) Consideration of the use of Multi-Criteria Decision Analysis in drug harm decision making. Available at:report?view=Binary
Babor T., Caulkins J., Edwards G., Foxcroft D., Humphreys K., Medina Mora M., Obot I., Rehm J., Reuter P., Room R., Rossow I. and Strang J.. (2010) Drug Policy and the Public Good. Oxford, Oxford University Press.
Brandt S.D., Sumnall H.R., Measham F., Cole J. (2010) The confusing case of NRG-1. British Medical Journal 341: c3564.
Brown, D. (2010) “FDA warns dietary supplement makers to police themselves on drugs in products” Washington Post, December 15
Byrd, S. (2011) “Officials: 'Bath salts' are growing drug problem” Associated Press, January 23
Cameron, L. (2006) Environmental Risk Management in New Zealand – Is There Scope to
Apply A More Generic Framework? New Zealand Policy Perspectives Paper. Wellington, NZ.
Campbell et al. (1973) Comparison of the effects of 1-benzylpiperazine and dexamphetamine in former addicts. European Journal of Clinical Pharmacology 6: 170-176.
Caulkins, J.P. and Pacula, R. (2006) Marijuana markets: inferences from reports by the household population. Journal of Drug Issues 36(1): 173-200.
Caulkins, J.P. and Coulson, C. (2011) “To Prohibit or Not to Prohibit: How Well Do We Decide?” unpublished paper, Carnegie-Mellon University
Cawley, J., Avery, R., Eisenberg, M (2011) “The Effect of Advertising and Deceptive Advertising on Consumption: the Case of Over-the-Counter Weight Loss Products” unpublished paper
Cook, P.J. (2007) Paying the Tab: The Costs and Benefits of Alcohol Control. Princeton: Princeton University Press
Coulson, C. and J. Caulkins (2011) “Scheduling of Newly Emerging Drugs: A Critical Review Of Decisions Over 40 Years” unpublished paper, Carnegie-Mellon University
Drug Enforcement Administration (2003) Drug Scheduling Actions - 2003 - Placement of 2,5-Dimethoxy-4- (n)-propylthiophenethylamine, N-Benzylpiperazine and 1-(3- Trifluoromethylphenyl)pip.
DEA (2010) DEA Moves to Emergency Control Synthetic Marijuana. Available at:
The Economist (2010) Legal Highs in Poland; Questions of Substance. October 8
Eichler H., Pignatti F., Flamion B., Leufkens H. and Breckenridge A. (2008) Balancing early market access to new drugs with the need for benefit/risk data: a mounting dilemma, Nature Reviews Drug Discovery 7: 818-826.
EMCDDA (2007) Risk Assessment Report of a New Psychoactive Substance: 1- Benzylpiperazine (BZP) Lisbon.
EMCDDA (2009) EMCDDA Action on New Drugs Briefing Paper: Understanding the ‘Spice’ Phenomenon. Lisbon.
EMCDDA (2009a) Risk Assessment of New Psychoactive Substances: Operating Guidelines. Lisbon.
EMCDDA (2009b) Legal Responses to New Psychoactive Substances in Europe. Lisbon.
Europol and EMCDDA (2010) EMCDDA-Europol 2009 Annual Report on the Implementation of Council Decision 2005/387/JHA
EACD (2006) Advice to the Minister on: Benzylpiperazine (BZP) Expert Advisory Committee on Drugs: Welllington, NZ.
EACD (2007) Further EACD Advice on Benzylpiperazine (BZP) and Related Substances. Expert Advisory Committee on Drugs :Welllington, NZ.
FTC (2002) Weight Loss Advertizing: An Analysis of Trends Washington, DC
Freye N. (2009) Pharmacology and Abuse of Cocaine, Amphetamines, Ecstasy and Related Designer Drugs: A comprehensive review on their mode of action, treatment of abuse and intoxication. New York, NY: Springer.
Gee P., Richardson S., Woltersdorf W. and Moore, G (2005) Toxic effects of BZPbased herbal party pills in humans: prospective study in Christchurch, New Zealand. New Zealand Medical Journal 118: 1227.
GAO (2002) “Dietary Supplements for Weight Loss: Limited Federal Oversight Has Focused More on Marketing than on Safety.” GAO-02-985T.
Griffiths, et al. (2010) How globalization and market innovation challenge how we think about and respond to drug use: ‘Spice’ a case study. Addiction 105: 951-953
Grimm R. (2009) This is Your Nation on Drugs. Hoboken, NJ: Wiley
Home Office Crime and Drug Strategy Directorate (2006) Review of the UK’s Drug Classification System: A Public Consultation.
Hughes B. and Blidaru T. (2009) Legal Responses to New Substances in Europe. EMCDDA,
Kalant H. (2010) Drug classification: science, politics, both or neither? Addiction 105: 1146–1149.
Kau G. (2008) Flashback to the Federal Analog Act of 1986: Mixing Rules and Standards in the Cauldron. University of Pennsylvania Law Review 156: 1077-1115.
Kay A. (2002) The agony of Ecstasy: reconsidering the punitive approach to United States drug policy. Fordham Urban Law Journal 29: 2133, 2163-66.
Marre O. (2008) The Right Stuff? The Observer. November 16.
Measham F., Moore K., Newcombe R., and Welch Z. (2010) Tweaking, Bombing, Dabbing and Stockpiling: the emergence of mephedrone and the perversity of prohibition. Drugs and Alcohol Today 10(1): 14-21.
New Zealand Drug Foundation (2007) Misuse of Drugs (Classification of BZP) Amendment Bill. Comments to Parliament. Wellington, NZ.
New Zealand Law Commission (2010) Controlling and Regulating Drugs. Wellington, NZ.
Nutt D., King L.A., Saulsbury W. and Blakemore C. (2007) Development of a rational scale to assess the harm of drugs of potential misuse. Lancet 369: 1047-1053.
Nutt, D. (2009) “Estimating Drug Harms: A Risky Business?“ The Eve Saville Lecture, The Centre for Crime and Justice Studies (CCJS) at King’s College, London
Nutt, D., King, L.A., and L.D. Phillips (2010) Drug Harms in the UK: A multicriteria decision analysis. Lancet 376(9752): 1558-1565.
Nutt D. (2010) Banning naphyrone will get us nowhere. The Guardian. July 9. Available at: acmd-recommendation.
Paoli L. and Reuter P. (2008) Drug trafficking and ethnic minorities in Europe. European Journal of Criminology 5: 13-37
Pillitteri, J.L., S. Shiffman, J.M. Rohay, A.M. Harkins, S.L. Burton, and T.A. Wadden.
2008. “Use of Dietary Supplements for Weight Loss in the United States: Results of a National Survey.” Obesity 16: 790-796
Pollard, S. (2001) An overview of the use of risk assessment for environmental regulation in the UK – key drivers and regulatory initiatives. Risk Decision and Policy 6(1) 33-46
Sedefov, R. and D. Lopez (2009) The European early warning system on new drugs a powerpoint presentation by the European Monitoring Center on Drugs and Drug Abuse
nce2009.pdf
Sheridan J., Butler R., Wilkins C. and Russell B. (2007) Legal piperazine-containing party pills: a new trend in substance abuse. Drug and Alcohol Review 26: 335-343
Sheridan J. and Butler R. (2010) ‘They’re legal so they’re safe, right?’ What did the legal status of BZP-party pills mean to young people in New Zealand. International J. Drug Policy 21: 77-81
Stratton, K., Shetty, P, Wallace, R. and S. Bondurant (eds.) 2001 Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Washington: National Academy Press.
Wilkins C., Girling M., Sweetsur P., Huckle T., and Huakau J. (2006) Legal party pill use in New Zealand: Prevalence of use, availability, health harms and ‘gateway effects’ of benzylpiperazine (BZP) and trifluorophenylmethylpiperazine (TFMPP). Auckland: Massey University Centre for Social and Health Outcomes Research and Evaluation (SHORE).
Winstock A., Marsden J. and Mitcheson L. (2010) What should be done about mephedrone? British Medical Journal 340: 1605
Winstock A. and Ramsey J.D. (2010) Legal highs and the challenges for Policy Makers. Addiction 105: 1685-1687
© UK Drug Policy Commission (UKDPC), May 2011.
This briefing is available at:
www.ukdpc.org.uk/publications.shtml
| Next > |
|---|












