| Articles - Treatment |
Drug Abuse
ON THE EVALUATION OF TREATMENTS FOR NARCOTICS ADDICTION
Vincent P. Dole* Burton Singer*
This study is concerned with the domain of applicability of randomized clinical trials. For evaluation of well-defined treatments of acute diseases over limited periods of time, the randomized trial technique is unquestionably the best. However, in the field of chronic diseases (as illustrated by drug addiction) the physician's responsibility extends over periods of years, and his judgements involve consideration of many contingent factors which vary in the course of the disease. In this domain, randomized clinical trials, however ambitious in design, give only partial guidance. Observational data therefore must be used if treatment is to be optimized for individual patients.
Two randomized trials in the treatment of narcotics addiction - one testing methadone and the other naltrexone - are reviewed, with comments on their conclusions and limitations.
*Vincent P. Dole is a Professor and Senior Physician to the Hospital, Rockefeller University. Member of the Association of American Physicians, the Institute of Medicine, the American Academy of Arts and Sciences, the National Academy of Sciences, and of speciality societies. Co-developer (with Marie Nyswander) of the Methadone Maintenance Treatment Program for rehabilitation of otherwise intractable narcotics addicts.
*Burton Singer is Professor of Mathematical Statistics at Columbia University and Adjunct Professor, Rockefeller University. He is a member of American Statistical Association, Psychometric Society, AAAS and is chairman of the Social Science Research Council's Committee on the Methodology of Longitudinal Research. His current research includes modeling of malarial transmission from WHO longitudinal surveys and development of methodology for the evaluation of treatments for chronic diseases.
A friendly critic of methadone maintenance programs recently observed that much of today's controversy could have been avoided it the originators had conducted randomized clinical trials. It is of course too late to start again, but even with the benefit of hindsight his advice seems overly optimistic. The controversies over this mode of treatment stem almost entirely from philosophical differences objections to the "substitution of one drug for another" -and not from doubts about the pharmacological safety and efficacy of methadone when it is dispensed under medical supervision. During the past twelve years many thousands of previously intractable addicts have been socially rehabilitated by maintenance programs (Newman, 1977). They now function in good health as normal members of their communities while receiving a daily dose of methadone. No alternative drug-free modality has had comparable success with this group of chronic addicts. Nevertheless, resistance to maintenance treatment remains strong and the objectors are politically influential. At present, the treatment as a rehabilitation modality is being extinguished by political pressures and over-regulation (Dole and Nyswander, 1976).
However, apart from philosophical difficulties, the issue raised by the critic is quite valid. The original work on methadone maintenance treatment was unsystematic and intuitive (Dole and Nyswander, 1965; Dole and Nyswander, 1967). As new and potentially better methods are developed for treatment of addicts, we hope that they can be tested more efficiently. It is not obvious how this can be achieved.
Short-term pharmacological effects of a new medication can be examined by randomized trial, but the conclusions are usually of limited value except when they are negative. Randomized trials provide little guidance in optimizing long-term, multivariate programs. This is especially true of a rehabilitation process in which a medication, such as methadone, may be necessary but not sufficient for a good outcome (Dole, et al. 1968). In the present discussion we examine the problems of evaluating treatments for narcotic addiction-and for other chronic diseases-to see what general principles may be involved in limiting the domain of questions that are answerable by randomized trials. We are especially concerned with the problem of defining the best possible long-term treatments for patients with incurable conditions.
The fundamental problem in all randomized trials is complementarity of precision and scope. When a narrow question is addressed to a restricted population, generality is in doubt. The relevance of the answer to the unstudied majority remains undetermined unless it can be assumed that the variate examined is so dominant as to have the same effect under different conditions, and the trial provides no test of this assumption. Conversely, if the sample is elaborately stratified to define the scope of a treatment, replications are lost. Moreover, in a multivariate treatment there is no reason to believe that the conditions of study will produce the best possible result. On the contrary, the standardized schedules necessary for controlled trials cannot provide optimal responses to complicating problems, or even allow the small flexibilities that become important when treatment is prolonged.
In principle, a contingent variable or special service that might affect outcome can be included in the experimental design, if anticipated. Theoretically, if the error variance per experimental unit remains constant with increase in size of the study, and if both the supply of patients and financial resources are unlimited, any number of contingent variables could be examined. In fact, however, above a certain size (which depends on the nature of the trial) either the error variance per experimental unit increases sharply or the study is limited by cost (Forrest et al., 1969; Smith, 1977).
To illustrate these general remarks, consider the demographic variates that might affect response of addicts to a treatment program. The age of the subjects and duration of addiction, the sex, ethnic group, neighborhood of residence, educational level, family status, employment, criminal record, previous treatment, and presence of additional problems such as alcoholism or other kinds of drug abuse, have a potential effect on patients' motivation to enter the study, on their willingness to continue for a prescribed time and on their outcome after discharge. With a simple scaling of these variates (4 age groups, 2 sexes, 4 ethnic, etc.), over one million combinations exist. This number of course must be reduced by excluding some categories from the study and ignoring other differences. A controlled trial of addicts necessarily is limited to a sub-group of the population.
For short studies, lasting less than about six weeks, some of the above mentioned problems disappear. Many patients can be persuaded to accept inconveniences for a few weeks, and variables in the environment can be held reasonably constant. But chronic diseases cause disability over periods of years. In the long term many patients with chronic diseases drop out of treatment, while others remit spontaneously or die. Treatment programs are likely to change with time, perhaps essentially, despite initial plans for a controlled study. In practical terms, a few people might persist in a study for several years, and a thousand people might be kept on a fixed routine for a month or two, but it would be unrealistic to expect a large number of subjects to follow a rigid schedule over a period of time comparable to the course of a chronic disease.
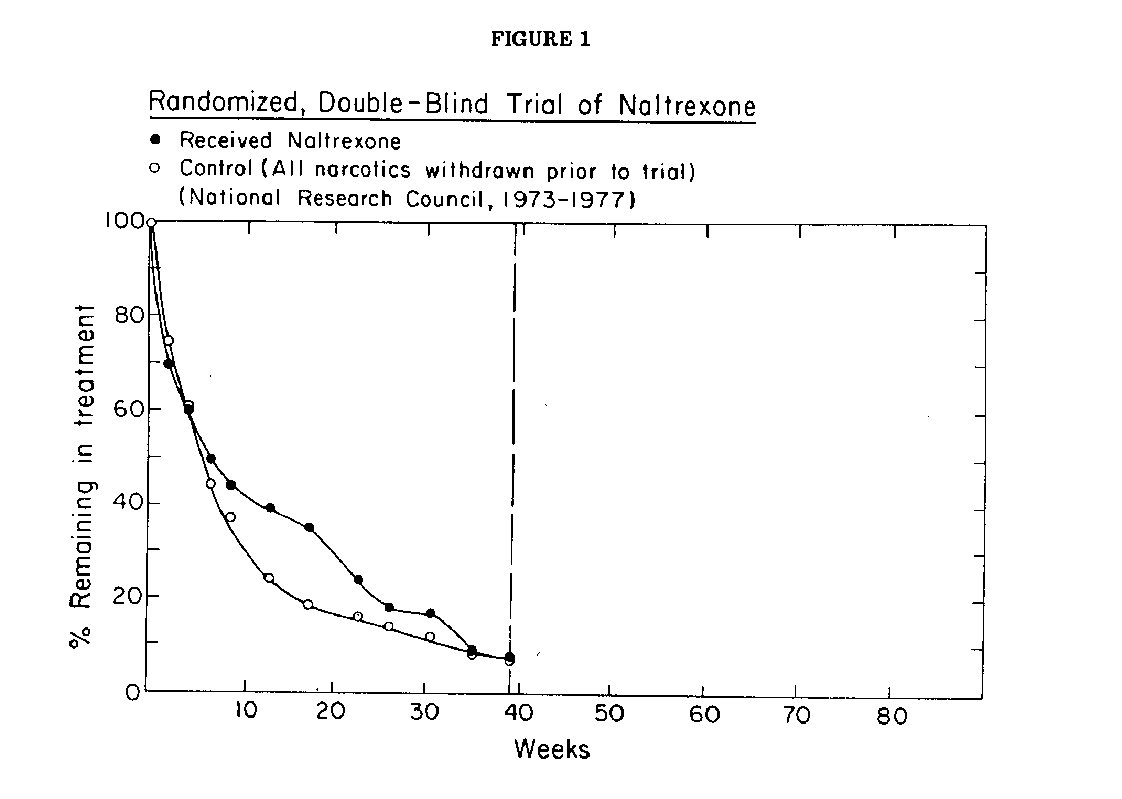
A recently published trial of naltrexone (National Research Council, 1977) illustrates some of these problems. Designed by statisticians with the advice of experts in the field of addiction, conducted under the jurisdiction of the Committee on Problems of Drug Dependence of the National Research Council, the study plan included the best statistical techniques of random assignment, double blind administration of the drug, and objective evaluations of outcome. The criteria for selection of subjects and their number, the services to be provided by participating clinics, the duration of the trial and the length of post-treatment followup were specified in advance by the planning team. In all respects the planning of this study seemed exceptionally rigorous. It was conceived not only as a test of the drug naltrexone, but also as a demonstration of how chemotherapy for narcotics addiction should be evaluated.
At the beginning of the study, the clinicians screened a total of 735 subjects being treated in five different clinics; of these 597 met the protocol requirements. Unfortunately only 192 (32%) of the eligibles were willing to enter the study, despite its relatively short length (9 months of treatment and 6 months of post-treatment followup). As the trial progressed both test and control subjects dropped out at an approximately equal rates. (Fig 1) By the end of the planned nine months of testing, only 13 (8 naltrexone, 5 control) of the starters, had completed the trial. The scheduled observations on drug abuse and psychosocial response to treatment nevertheless were diligently made on the few subjects who continued in the study. No difference was detected between the test and control group. No followup data were reported.
It would seem from these results that the drug tested was worthless, but the investigators were more cautious in their interpretation. In their final report they generously attributed the disappointing outcome of the study to deficiencies in their planning rather than to the drug, explaining that "This study was not expected to lead to definitive conclusions concerning these three points . . , " (i.e., efficacy, acceptability and toxicity). They felt however that " . . . this preliminary study demonstrated the feasiability of a controlled, double masked study of the clinical efficacy of a narcotic antagonist." Conceivably the drug might be useful under different conditions, but the results of this trial have not encouraged these or other investigators to continue the work. The known costs of the trial included a budget obtained from the National Institute on Drug Abuse for utilization of facilities in five clinics for 2 years, plus $487,400 of overhead expenditure for planning and management of the study. The unobserved costs were the relapses and deaths of an unspecified number of addicts who returned to the streets after having received ineffective treatment. This ambitious effort, reported in great detail, deserves careful study by advocates of controlled clinical trials in chronic disease.
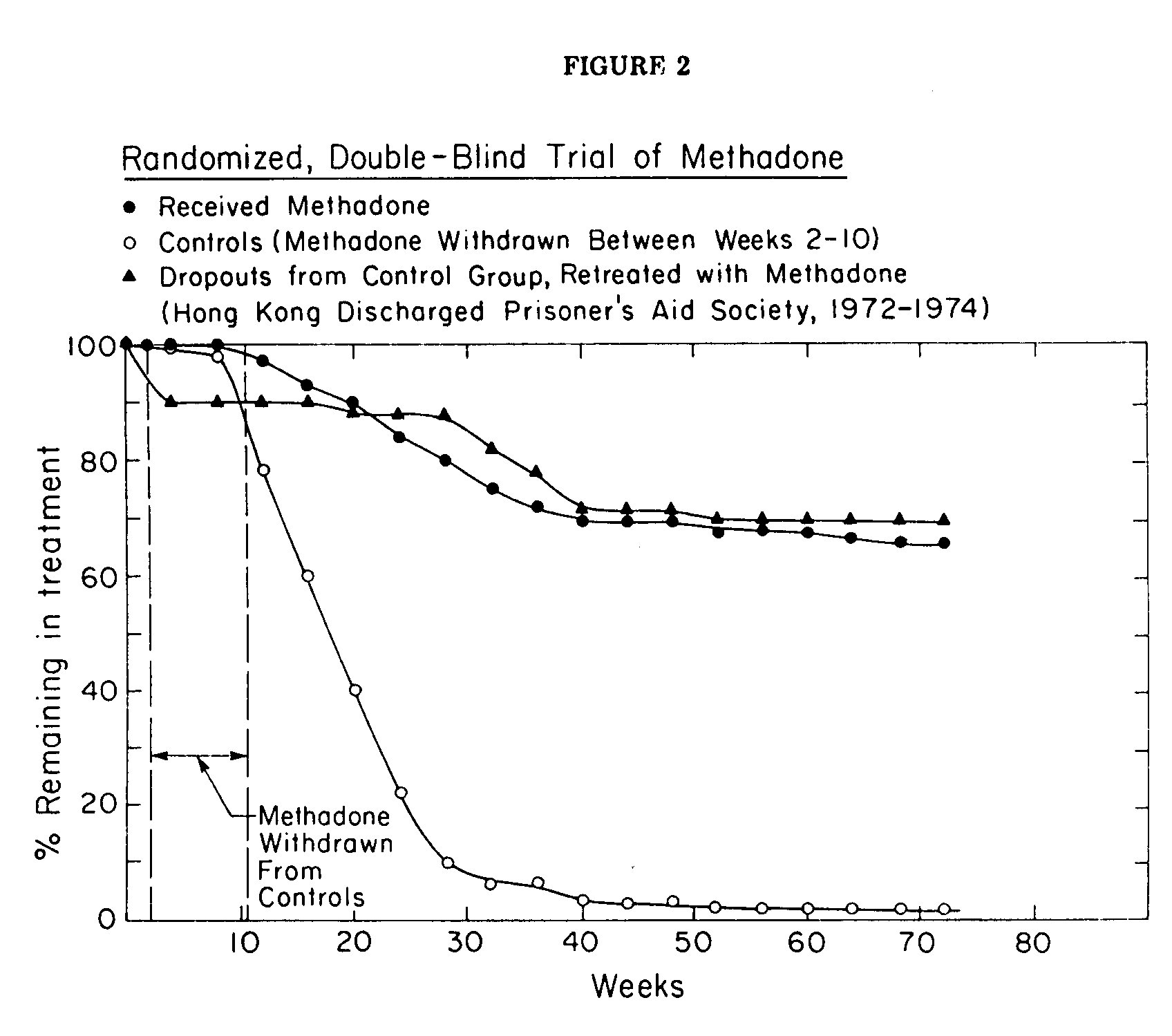
A randomized study of methadone maintenance treatment, reported from Hong Kong (The Hong Kong Discharged Prisoner's Air Society), showed more encouraging results. As in the naltrexone trial, participation was voluntary, with the important difference that dropouts (persons who failed to report for six weeks) were located in the community and, if possible, persuaded to return for more treatment. The sample for the study was obtained by taking 100 consecutive addicts who applied for treatment in the clinic. These were pair matched by age, addiction history, criminal record and family status to yield 50 similar pairs. One member of each pair was assigned randomly to methadone and the other to placebo treatment, the assignment being known only to the pharmacist who prepared the medications.
All subjects were stabilized initially on methadone (60 mg/day). After this preliminary two-week period, the medication was withdrawn from the control group at a rate of I mg/day until they reached zero dose. Thereafter the control subjects received only a flavored placebo which was said to be indistinguishable in taste from methadone. The retention rates in the two groups were markedly different (Fig 2). After 9 months 75% of the methadone patients remained in the treatment while the census of the control group had fallen to less than 10%. By the end of the study (72 weeks), only I person (2%) remained in the control group while 62% (31/50) were continuing with methadone. Moreover, when the dropouts from the control group were retreated, this time with methadone, their retention rate and rehabilitation was the same as had been observed in the original methadone group. The employment rate for patients receiving methadone averaged about 80%; the subjects in the control group dropped out too fast for this or other social indices to be significant. Two thirds of the group receiving methadone were scored by staff workers (who were blind to the medication being received) as improved in the categories of financial support to family, concern towards family, behavior at home, communication with family members and acceptance by family. When the control group was re-treated with methadone their employment rate increased to 80%, and other measures of social rehabilitation became comparable to those of the original methadone group. The average maintenance dose of methadone in the trial was 86 mg/day.
Despite this demonstration of efficacy, the study failed to persuade clinicians in Hong Kong to use the dosage schedule that had been tested in the pilot study. Much lower doses are being used in their clinics, and the retention in these clinics is substantially below that of the controlled trial. It can be said that the trial was limited in scope, involving only 100 addicts in a special clinic. Whatever the merit of this argument, it is clear that randomized trials may not change previously held opinions. Similarly, the authors of the naltrexone trial, apparently convinced of the value of antagonist therapy on theoretical grounds, chose to repudiate their own study rather than acknowledge failure of the treatment. Of course the failure to persuade skeptics is not an argument against controlled clinical trials, but the experience can be taken as a warning that a randomized trial made at great cost and effort may have less effect than it deserves.
In general, the data available for evaluation of methadone treatment programs are observational and retrospective. Although less efficient than controlled studies, the large volume of clinical reports published over the past twelve years have clearly established two points: When the conditions of the original treatment have been replicated, results similar to those initially reported have been obtained by other clinicians treating chronic addicts (Newman, 1977). On the other hand, when the conditions have been radically changed, poor results have been obtained despite the administration of methadone. Factors associated with poor results are a shift of emphasis from rehabilitation to detoxification as the goal of treatment, a staff hostile to the treatment that it is providing, contempt for the patients as inadequate and untrustworthy individuals, rigid and punitive rules, requirement of daily attendance, and low dosage (Dole and Nyswander, 1976). Methadone cannot guarantee a good outcome under unfavorable conditions. It may be necessary, but obviously is not sufficient for rehabilitation (Dole and Nyswander, 1967).
These problems are not unique to methadone. The variety of symptoms in any chronic disease and the interactions between medications, supportive services and attitudes of the professional staff defy systematic analysis. When the period of concern extends to the entire course of disease--as it must for physicians treating real patients--the number of possible disease careers increases beyond hope of detailed documentation. Although randomized clinical trials can provide guidance in eliminating worthless procedures (if the results are believed), the technique is of little value in optimizing treatment of chronic disease.
Traditionally, an experienced physician is guided by general rules and an intuitive feeling of what treatment may be appropriate for individual patients. like an actuary, he can use historical data on the outcome of various treatments for general predictions but cannot anticipate the details of any specific case. Perhaps his recording of experience could be made more systematic, but his problem is much deeper than efficiency in data collection.
The analogue to the clinician's task is a game with imperfect information in which the variants are too numerous for exhaustive analysis. In place of a solution, an analyst of complex games can only seek a strategy to maximize the chance of winning. Similarly, in medical practice the best treatment for a particular patient may be defined as the one among all available alternatives that has the highest probability of a favorable outcome. Randomized clinical trials can provide guidance in selecting treatments and avoiding useless procedures, but since they are never exhaustive in scope, the evidence from them is incomplete. The clinician, therefore, must also draw upon clinical experience. While there is an inevitable loss of rigor in passing from randomized trials to observational data, the analyst must recognize the need of the clinician for a wide range of experience to make wise judgements in particular cases. The clinician needs effective strategies that utilize all available data in reaching these clinical decisions.
REFERENCES
Dole, V. P., Nyswander, M. E. 1965 A Medical Treatment for Diacetylmorphine (Heroin) Addiction - JAMA . 193:646.
Dole, V. P., Nyswander, M. E. 1967 Rehabilitation of the Street Addict - Arch. Environ Health 14:477.
Dole, V. P., Nyswander, M. E., Warner, A. 1968 Successful Treatment of 7 50 Criminal Addicts -JAM A 206:27 08.
Dole, V. P., Nyswander, M. E. 1976 Methadone Maintenance Treatment: A Ten Year Perspective - JAMA 235:2117.
Forrest, W. H., Jr., Brown, B. W., Jr., Bellville, J. W. 1969 The National Halothane Study. Chapter 11-1. Approaches to the Study: Randomized Trial or a Study of Past Data - U.S. Dept. HEW.
Hong Kong Discharged Prisoner's Aid Society 1978 Methadone Maintenance Pilot Scheme: Final report on the Three-Year Experiment, 6 December 1972 to 6 December 1975. Hong Kong. (New Life Printing Press)
National institute on Drug Abuse 1977 Pilot Studies in the Clinical Evaluation of Narcotic Antogonists. Final Report. National Research Council, Washington, D.C.
Newman, R. G. 1977 Methadone Treatment in Narcotic Addiction - Academic Press.
Smith, W. M. 1977 Detection of Hypertensive Populations for Study and Intervention, p. 1155, in Hypertension (Genest et al, Editors) - McGraw Hill.












