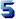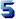|
In response to the wishes expressed by the Madagascar Delegation at the 27th Session of the United Nations Commission on Narcotic Drugs and pursuant to a resolution adopted at this Session, the UN Narcotics Laboratory decided on 7th June 1977 to investigate the chemical properties and physiological effects of khat (Arabian tea) grown in Madagascar. For this purpose, two experts from the UN Laboratory Drs. I. Chilov and K. Szendrei, arrived in Madagascar on 15th November 1977, where they remained until 5th December 1977. The purpose of the mission was the collection of specimens of khat for chemical analysis.
Several months later, it was announced that a meeting of a group of experts would be held at Antananarivo in order to collate all available data on the chemistry of khat and to determine the orientation of future research in this field. The UN Laboratory invited the National Centre for Pharmaceutical Research to attend the meeting, which was held at the Ministry for Foreign Affairs from 27th November, to 1st December 1978.
In the light of previous research on khat, we oriented our studies along two main lines:
- Separation of the constituents of khat;
- hemisynthesis of cathinone.
The present paper deals with these studies, which were presented at the meeting of the group of experts in 1978.
Khat (Catha edulis Forsk, Celastraceae) is a shrub native to certain East African countries and to the southern region of the Arabian peninsula. It is widely cultivated in the north of Madagascar, where it was introduced in about 1940. The inhabitants of these regions chew the young leaves and fresh tips of the plant, from which they obtain stimulant effects. Two varieties of khat found in Madagascar are:
- the red variety;
- the white variety.
Study of the chemical constituents of khat began as long ago as 1887. The results obtained until quite recently show that khat is composed chiefly of two basic-type constituents possessing stimulant properties:
- phenylalkylamine such as cathine (or nor-pseudoephedrine);
- polyester-type alkaloids of high molecular mass.
Other constituents, such as amino acids, tannins, flavones, itols, essential oils and ascorbic acid, have also been mentioned. However, the results obtained are somewhat ambiguous and far from conclusive. Recent extensive studies by the United Nations Narcotics Laboratory have revealed the existence of cathinone - another, highly unstable phenylalkylamine-type compound, neutral substances, and - in collaboration with L. Crombie - other polyester alkaloids.
The method used to separate the various constituents of khat consists essentially in isolation of groups along a pH gradient. Despite a certain selectivity of this technique, the complexity of the separated groups is such that it is impossible to identify the individual constituents by either thin-layer or gas chromatography. Moreover, certain constituents of khat, particularly the basic compounds, are relatively unstable in acid or alkaline medium, and some may be partly degraded by the pH gradient procedure. This technique presents a further drawback, namely that extraction of the basic compounds, some of which are water-soluble, is never complete and hence accurate quantitative determination of the active principles present is impossible.
A notable feature of the chemical constituents of khat is the wide range of molecular mass, from about 1400 for polyester-type alkaloids to 150 for the phenylalkylamines. We therefore attempted to separate the different constituents by filtration on sephadex gel which, being chemically inert to a number of different substances, is well suited to separation of the constituents of khat. Further advantages of this technique are its relative rapidity, its efficiency and its high reproducibility.
Freshly picked leaves of khat are extracted by maceration in alcohol. When evaporated to dryness under reduced pressure, the alcoholic solution leaves a green residue which is used for subsequent analyses. The raw extract thus obtained is dissolved in a 30/70 chlorofrom/methanol mixture which also serves as the eluent solution. The solution obtained is filtered to remove the precipitate, concentrated and then chromatographed on sephadex gel LH2O, previously swollen with the 30/70 chloroform/methanol mixture. Each fraction is analyzed by silica thin-layer chromatography in two eluent systems: 80/20/10 benzene/ethyl acetate/methanol and 5/3/3/1 ethyl acetate/butanone/formic acid/water. Three developing reagents are used: Dragendorff, ninhydrine, and 50% sulphuric acid, followed by heating in an oven at 120 °C.
The 80/20/10 benzene/ethyl acetate/methanol eluent system confirms the presence of the following substances:
- the polyester-type alkaloids, which separate out in the first fractions. Comparison of the Rf values with standard samples of cathedulin-2 and cathedulin-11 demonstrates conclusively the presence of these alkaloids in both varieties of khat ;
- a triterpenic heteroside which appears to be characteristic of the red variety but is not present in the white variety;
- free triterpenes.
The 5/3/3/1 ethyl acetate/butanone/formic acid/water eluent system reveals three types of constituents:
- two terpenic heterosides common to both species.
When isolated in the pure state by column chromatography using silica as the absorbent, they give a positive Liebermann-Burchard reaction;
- the phenylalkylamine-type compounds and the amino acids. It should noted that the raw extract used, obtained by extraction of old leaves of khat, contained only traces of cathinone;
- the flavonoid compounds are clearly in evidence and well separated at the end of the column. The red variety is far richer in phenolic compounds, some of which are certainly responsible for the red colouration of the stems and veins.
This study demonstrates that the various constituents of khat may be isolated by filtration on sephadex gel LH2O, which should be carried out prior to chromatographic separation.
The second part of our research deals with the hemi-synthesis of cathinone 1:
Structurally, cathinone 1 may be considered to consist of two components:
- a benzenic component,
- an L-alanine residue.
We exploited this fact when carrying out the hemisynthesis of cathinone 1. First, the amine function of the L-alanine 2 is protected by benzylchloroformiate. The reaction is carried out at 0 °C in a 2N solution of Na2CO3. N-carbobenzoyl-L-alanine 3 is precipitated following acidification of the reactant medium to pH 2. The compound 3 is treated with PCI5 in anhydrous ether at 0 °C, yielding the corresponding acid chloride. After evaporation of the ether under reduced pressure, the compound 4 is coupled directly with anhydrous benzene in the presence of anhydrous AICI3 to form N-carbo-benzoxy-cathinone 5 with a 20% yield. Hydrogenolysis of compound 5 produces cathinone 1.
The advantage of this hemisynthesis is that the asymmetrical alanine centre remains virtually intact throughout the various steps. In 1982, Merck published a similar hemisynthesis of amino-2 propiophenone.
We intend to pursue the hemisynthesis of hydroxylated derivatives of cathinone, since such compounds may occur naturally in khat.
|