CENTRAL NERVOUS EFFECTS OF LSD-25
| Books - The Psychedelics |
Drug Abuse
CENTRAL NERVOUS EFFECTS OF LSD-25
WERNER P. KOELLA, M.D.
In the majority of chapters of this book, the psychotomimetic, psychedelic, and hallucinogenic effects of LSD-25, and in this connection, the possible beneficial and damaging actions of this drug, have been described and discussed. Such considerations belong in the realm of the psychiatrist, psychologist, sociologist, geneticist, and possibly even the theologian and student of mysticism. In the present chapter, we shall look at LSD-25 from the point of view of the neurophysiologist and neuropharmacologist, investigators who are interested in understanding how the central nervous system (CNS) works and how drugs act on this substrate.
One can assume that every behavior of man and animal, such as reflex movements, instinctive patterns, learned actions and reactions, voluntary behavior, various states of vigilance, as well as thinking, moods, and recall of memory bits, is the manifestation of a particular and specific time-intensity-space pattern of activity in the nerve cells. About five to ten billion of these cells, together with an even greater number of supporting cells (the glial elements), make up the central nervous system of higher animals and man. During the past few years, neurophysiologists, using electrical recording techniques, have been able to detect, and to determine in their quantitative and qualitative aspects, correlates in nervous activity of such behavioral phenomena as sleep, resting, waking, arousal, the orienting reflex, memory traces, and many others.
It can be assumed further that abnormal behavior such as is encountered in mental disease is the manifestation of an aberrant time-intensity-space pattern of activity in the nervous system. One may then postulate that the abnormal behavior produced by such drugs as LSD-25, which in some aspects resembles the behavior exhibited by mentally ill people, is also the manifestation of similarly aberrant nervous activity, and that—to go even further—this abnormal activity is the direct consequence of the drug effect.
Finally, it is not unlikely that LSD-25 and similar drugs induce in the CNS of experimental animals such changes in nervous activity. Due to the certainly less complex "personality" of such animals, however, abnormalities in nervous activity may not manifest themselves by discrete abnormalities in behavior. But such abnormalities can, with some chance for success, be studied with the tools of the neurophysiologist.
The neuropharmacologists have in the past ten years or so intensively studied the effects of LSD-25 and similar drugs on various aspects of brain function. And indeed, in a number of instances, some insight has been gained into the way in which behavioral effects of the drugs may relate to abnormal nervous activity and reactivity. While this is just a beginning of a relatively new scientific field, it is hoped that, with an even greater effort and with refinement of our techniques, we shall, in not too many years, be able to state with some certainty what nervous circuits in the brain do deviate from their normal activity patterns to bring about behavioral disturbances.
In the following, we describe and discuss some of the work done in this field.
The Effect of LSD-2 g on the Electroencephalogram
The electroencephalogram, or EEG, is the record of the electrical brain waves. It is obtained by means of electrodes (usually metal leads) placed on the surface of the brain, or aimed into particular structures of the interior of the brain. In routine clinical electroencephalography in man, the electrodes are attached to the scalp. The electrical potential-changes occurring in these recording sites are amplified about one million times and written out with ink on moving paper. The recorded EEG is a more or less irregular wavy line that allows us to distinguish oscillations of various frequencies. A discussion of the origin of these brain waves is beyond the scope of this chapter, and indeed, physiologists still do not understand this problem too well. It is of importance, though, to point out that these brain waves vary with the functional state of the organism, and, of less importance here, that they signal, by abnormal wave forms and "spikes," pathological processes in the brain. As to the former aspect, it was recognized quite early in the development of the electroencephalographic technique that fundamental changes occur in the appearance of the EEG, derived from the scalp in man and from the surface of the brain in animals, as the subjects shift from the aroused attentive state to quietly resting waking, to drowsiness, and to light and then deep sleep. In the aroused state, the EEG is characterized by an irregular low-voltage pattern exhibiting frequencies of about fifteen to thirty cycles per second, the so-called beta waves. In resting waking there are, particularly in the posterior part of the brain, the typical alpha waves, i.e., rather regular oscillations of about eight to twelve per second (of somewhat lower frequencies in the cat). During the drowsy, or "floating," state, the alphas gradually disappear and are replaced again by a beta pattern. With the onset of actual sleep, the EEG is characterized first by the occurrence of bursts of ten-to-fourteen-per-second waves that wax and wane so as to produce a spindlelike envelope; hence we refer to these as sleep spindles. With the shift to deeper states of sleep, high-voltage, very slow waves (delta waves, one to three per second) dominate the picture. More recently it has been found that subjects can be sound asleep and still show at times episodes of arousal patterns in the EEG (i.e., low-voltage beta waves); these episodes are accompanied by rapid eye movements (REMs). When human subjects are awakened during these REM periods, they almost invariably report that they have been dreaming, whereas they do not do so when awakened from a slow sleep episode. It is for this reason that we think today that during these REM periods, or periods of "paradoxical" or "activated" sleep, we dream. These REM episodes occur in man about every ninety minutes, in the cat about every thirty minutes. Figure i shows some typical records obtained from a "chronic" freely moving cat. With the help of such tracings, together with continuous observation, the actual level of "vigilance" can be diagnosed with a high degree of reliability. The experiments discussed below are based on such techniques.
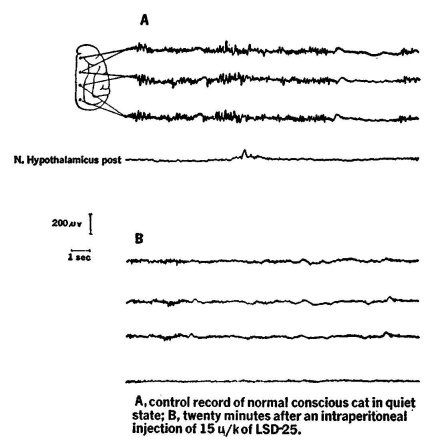
Figure i. Records taken from a cat chronically prepared with electrodes to record: the eye movements (EOG), the electroencephalogram from both sides of the skull (EEG), and the activity of the neck muscles (NMG). The animal was kept in an air-conditioned, sound-attenuated room supplied with a one-way mirror for observation. The records were taken while the animal was awake and active (top left), awake and resting (top right—note alpha waves), just dropped off to sleep (middle left —note "spindles"), in deeper sleep (middle right—note slow waves in EEG); in still sounder sleep (bottom left—note very slow waves), and in paradoxical sleep (bottom right—arousal pattern in EEG). (Note eye movements in awake animal and in paradoxical sleep). Note also reduced electrical muscle activity in paradoxical sleep. Calibrations: vertical line at top middle = ioo microvolts (or o.000l volt), horizontal line = 1 second. Original from author's laboratory.
Bradley and Elkes x953) worked in chronic cats and monkeys, i.e., animals previously prepared with electrodes to record the EEG from the cerebral cortex. For the actual experiments, the animals were placed in special chambers that allowed observation as well as electrical recordings. LSD-25 (given by intraperitoneal injection) in doses ranging from fifteen to twenty-five micrograms per kilogram of body weight (one microgram being one millionth of a gram) induced within a few minutes after administration a shift in the EEG from the resting pattern to an arousal pattern that often lasted for several hours (figure 2). At the same time, the animals became restless and more alert, and their pupils became somewhat dilated. Similar effects were reported by Takagi and co-workers (1958) and Schwarz and collaborators (1956). Some of these investigators also administered LSD-25 intraventricularly, i.e., into the brain cavities, and found again that low doses of LSD-25 produced the arousal reaction. Larger doses of this drug, up to several milligrams (one milligram being one thousandth of a gram), however, tended to bring about bursts of abnormal-looking slow waves (Passouant et al., 1956; Vogt et al., 1957).
In the rabbit, low doses of LSD-25 also tended to produce an arousal pattern and to eliminate all electroencephalographic features of drowsiness and sleep for several hours (Rinaldi and Himwich, 1955). Again, elevation of the dose in this species reversed the picture by bringing about the appearance of slow waves and sleep spindles.
Rinkel and his co-workers (1952) studied the effect of LSD-25 on the EEG of human volunteers. They noted only slight changes, characterized by a small but distinct acceleration of the alpha-wave rhythm.
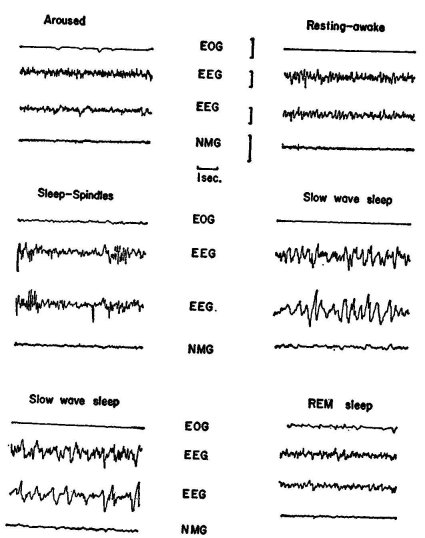
Figure 2. The effect of LSD-25 on the electroencephalogram of the normal unrestrained cat. Recording modes as indicated by connections and electrode position on cortex of cat brain in upper part of picture. Record 4 is derived from hypothalamus. A: (control) animal is in quiet waking state. Note alphalike waves in three cortical leads. B: records twenty minutes after LSD-25
Ag/kg) had been administered into peritoneal cavity, which leads to disappearance of alphas and to arousal pattern. Calibration in microvolts and seconds as indicated (From Bradley and Elkes, 1953). P. B. Bradley and J. Elkes, "The Effects of Sème Drugs on the Electrical Activity of the Brain," Brain, Vol. 80. 1957. Reprinted by permission of the authors and the publisher.
From such experimental evidence one may infer that in animals, and possibly in man, LSD-25 in low doses leads to an arousal state that can last for several hours. In view of the more recent information about paradoxical sleep, a somewhat different conclusion may be justified. An "arousal" pattern in the EEG does not necessarily signal the onset of arousal, but may just as well indicate the phase of paradoxical sleep. This suggests that in dream sleep, or REM sleep, the cerebral cortex is physiologically in a state similar to that present during arousal. The difference between sleep and waking seems to be due to differences in activity in other (particularly) brain areas. Since LSD-25 can produce visual hallucinations or illusions that can be somewhat similar to the type of images found in dreams, one may reason that LSD-25, by some still obscure mechanism, shifts the activity pattern in the cerebral cortex (and also some subcortical structures) in the direction of a state similar to that observed under physiological conditions during REM sleep. This interpretation is supported by Passouant and his collaborators' observation (1956) that LSD-25 produces in the cat behavior that looks very much like "visual hallucinatory troubles." The animals often lifted their paws as if to catch a fly; they would suddenly retreat with their eyes fixed on an imaginary source of danger, or they turned around as if to escape an attacker.
Of interest in this connection are findings on the influence of LSD-25 on sleep. As already indicated, one may, in a simplified fashion, subdivide the organism's life into three different stages of "being," alternating in a more or less regular manner: wakefulness, slow sleep (characterized by slow waves and spindles in the EEG), and paradoxical or dream sleep (characterized by low-voltage fast-wave EEG). In any particular individual (man or animal), each of these three stages occupies, under physiological conditions and in the absence of external modifying influences, a fairly constant proportion of the twenty-four-hour day. Thus, the influence of drugs and other factors can be monitored relatively easily in a well-equipped sleep laboratory.
Muzio and co-workers (1966) studied the effect of LSD-25 on the sleep of human volunteers. They found that with doses ranging from 6-4o p.,g total dose, the first two REM periods in the LSD-25 nights were prolonged, and that slow-wave sleep was often interrupted by brief REM periods. Hartmann (1967) also found an increase in total REM time and relative REM time (i.e., percentage of total sleep) in rats under the influence of LSD-25. These two experiments again support the idea that there is a relation between the functional changes that take place in the CNS during dream periods, on the one hand, and the changes induced by LSD-25, on the other.
Hobson (1964), on the other hand, found that LSD-25 in doses of 2 and 20 pg/kg of body weight reduced the paradoxical sleep in cats and made it less differentiated from slow-wave sleep. There were also more awakenings in these animals, and they tended to be more easily aroused by external stimuli. More work is needed on the fundamental mechanisms operating in the CNS both during REM sleep and during the particular state produced by LSD-25.
The Effect of LSD-25 on Sensory Transmission
Another method employed rather frequently by neuropharmacologists involves measurement of transmission in sensory systems by the so-called evoked potential technique. A sense organ, e.g. the eye or the ear, is stimulated by a light flash or a loudspeaker click, and an afferent volley of nerve impulses is produced. This volley travels first through peripheral sensory nerve fibers and then through the afferent systems in the CNS to reach finally the so-called projection area of the cerebral cortex.
In all sensory systems, this afferent pathway consists of at least three neurons (nerve cell and nerve fiber) arranged in a continuous chain. The transfer of nervous signals from one neuron to the next occurs in well-defined loci called synapses. The last subcortical synapse is located in the sensory relay nuclei of the thalamus, a part of the diencephalon located deep in the base of the brain.
When the volley of impulses reaches such relay locations or the projection area of the cerebral cortex, it leads to a more or less simultaneous transient excitation of a multitude of nerve cells. This excitatory focus manifests itself by an action potential or evoked potential that can be measured using electronic amplifiers and a fast-reacting recording device, usually a cathode-ray oscilloscope (similar in its technical principles to a TV set).
The amplitude of this evoked potential depends on the total amount of local nervous excitation. This, in turn, is related to the strength of peripheral stimulation and ease of transmission in the afferent channel. With a standard sensory stimulus applied to sense organs, or (as is done frequently) with a standard electrical stimulus applied to afferent nervous pathways, the amplitude of the evoked potential gives a measure of afferent conductance, or, to be more precise, of transmission at the synaptic sites of the afferent pathways including the synapses in the cortex of the brain. It is of importance to state that it is particularly these synaptic sites that are susceptible to drug action as well as to the modulating action of accessory neural systems.
Evarts and his collaborators (1955) investigated the influence of LSD-25 on transmission in the visual system of cats. These investigators not only were interested in the effect of this drug on the visual system as a whole, but, by an ingenious technique, they set out to study differential effects on the various synaptic sites in this afferent system.
To investigate drug action at the most peripheral sites, i.e., the light receptors and synapses in the retina, they stimulated the eyes of their (usually anesthetized) experimental animals with a light flash and measured the afferent volleys of nerve impulses by electrically recording from the optic nerve. They found that only very high doses of LSD-25 (5 mg/kg) were able to change these action potentials, which tended to become smaller. This indicated that the light receptors and/or the retinal synapses were relatively insensitive to this drug, and only under rather massive doses tended to react, and with decreased excitability.
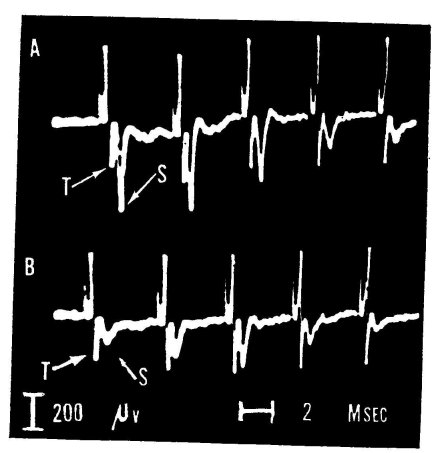
Figure 3. Influence of LSD-25 on synaptic transmission in lateral geniculate body of the cat. The optic nerve is electrically stimulated about 250 times per second, leading to the repetitive complex response seen in A and B. "T" points to the presynaptic wave arriving in the geniculate nucleus. After a delay of less than one millisecond, the nerve cells are synaptically excited and produce spike S. The deflections occurring prior to wave T are the so-called stimulus artifacts, i.e., the electrically conducted disturbance elicited by the electrical stimulus; they have no biological significance. A: control record of five responses out of a larger series. B: after 15 pg of LSD-25 had been given via the carotid artery. Note drastic reduction of postsynaptic spike, S, signaling impairment of synaptic transmission. (See Evarts et al., 1955-)
The most pronounced effect of LSD-25 was found on the synapses of the thalamic relay, as demonstrated in the following way: These investigators stimulated the optic nerves of anesthetized cats with electrical shocks and recorded action potentials by means of metal leads, insulated except for their tips and placed in the lateral geniculate body, the thalamic relay station of the visual system. The signals they recorded revealed two different waves in response to each stimulus. These signaled, as shown in figure 3, respectively the nerve impulse traveling from the stimulus site in the optic nerve to the geniculate body (the presynaptic spike = T) and the synaptic excitation produced by that afferent volley in the nerve-cell bodies of the geniculate nucleus (the postsynaptic spike = S). As is evident from figure 3, LSD-25, in rather low doses injected into the carotid artery, markedly depressed the postsynaptic spike, whereas it left the presynaptic spike unaltered. This clearly indicates that the drug had profoundly impaired synaptic transmission in the thalamic relay station but had not affected the excitability of the optic nerve fibers to electrical stimuli.
These scientists also investigated the effect of LSD-25 on the synapses of the cerebral cortical visual projection area. A response in this site was elicited by electrical stimulation of the optic radiation, i.e., the nerve fibers leading from the relay in the thalamus to the visual projection area in the posterior part of the cerebral cortex (figure 4). It was found that the response in the cortex to such stimuli even under high doses of LSD-25 was not depressed, but, rather, slightly enhanced. This indicated that the cortical synapses are relatively insensitive to the depressing effect of the drug and that they are made more, rather than less, excitable by LSD-25. In spite of the fact that LSD-25 in moderate doses failed to depress transmission in the retina and in the cortex, the drug-induced reduction in "gain" in the thalamic relay seemed to be sufficient to produce "behavioral blindness" in awake, freely moving cats (Evarts 1957) and monkeys (Evarts 1956).
The idea does not seem to be too farfetched to relate the impaired transmission in the visual pathway at the thalamic level, attended by increased excitability at the cortical sites, to the hallucinogenic action of LSD-25. One could indeed suggest that, in the absence of (or with reduced) visual input to the cortex, the somewhat more excitable visual cortical networks produce their own imagery, independent of what "meets the eye."
Purpura (1956) investigated the effect of LSD-25 on the visual and auditory system in cats that were supplied with recording and stimulating electrodes and, while non-anesthetized, were immobilized with curare-type drugs. He observed that LSD-25 in low doses (2-3op,g/kg given intravenously) enhanced the response evoked in the cortical projection areas by light flashes and loudspeaker clicks. Of interest also is Purpura's observation that larger doses of LSD-25, while still facilitating the response to photic stimuli, tended to depress the signal evoked by loudspeaker clicks. The question, of course, arises of how this investigator's results can be brought into accord with Evarts' observation; one would have to assume that the increase in cortical excitability more than compensates for the reduced transmission at the thalamic relay.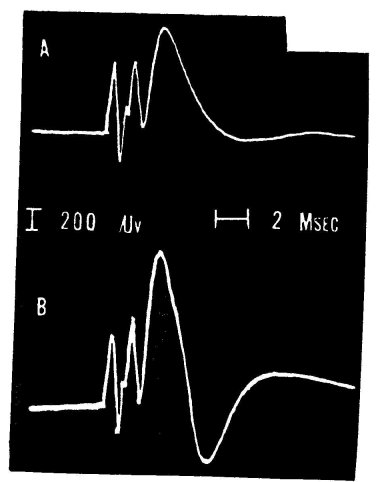
Figure 4. Effect of LSD-25 on synaptic transmission in the cerebral cortex of the cat. The response, recorded from the visual projection area, was produced by electrical shock to optic radiation. This response shows several typical deflections, the discussion of whose origin is beyond the scope of this paper. A: control, B: after injecting 1.5 mg of LSD-z5 into the carotid artery. Note particularly the increase of late components after the drug (from Evarts et al., 1955).
It seems that more work is needed to clarify this and other questions arising from experimental data obtained so far.
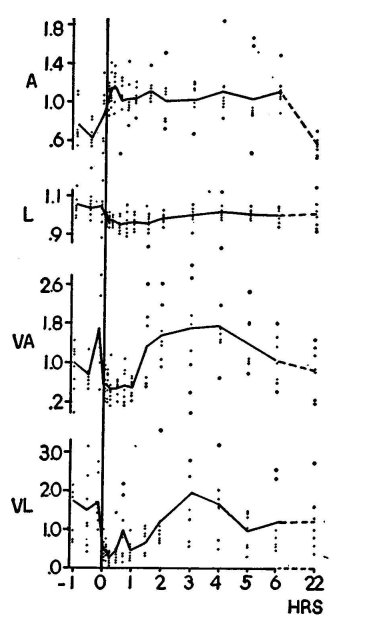
Figure 5. The effect of LSD-25 on the amplitude (A) and latency (L) of the cortical response to light flashes in the awake rabbit. Also shown are variability (expressed as variance) of the amplitude (VA) and of latency (VL). The measurements were done on the first downward component of the complex response shown in figure 6. Control (left of "o" hour) and effect of LSD-25 (50 pg total dose) were observed over several hours. The dots signify the readings (means of ten measurements of amplitude and latency) at each time for each of seven animals. In the case of variability, they indicate variance of the ten measurements. The full lines represent the means for all seven animals. Note slight protracted increase in amplitude and decrease in latency after LSD-25 (given at o hours). Note decrease and, after one to two hours, increase of variability of amplitude and latency. (From Koella and Wells, ro5o; reprinted by permission
of the American Journal of Physiology.)
Still, of interest in view of Evarts' results are the observations of Blough (1957). This author, using a rather intricate behavioral test arrangement, was able to measure the "absolute visual threshold" in pigeons. When the birds were given LSD-25 (either by mouth or injected into the peritoneal cavity), he invariably noted an increase in threshold, i.e., an impairment of vision, whereas motor and discriminative functions were not grossly disturbed. The rise in threshold could again be related to the reduction in transmission in the thalamus, as noted by Evarts.
The present author studied the effect of LSD-25 on visually evoked responses in rabbits (Koella and Wells, 1059). The animals were supplied with chronically implanted electrodes to record the signals in the visual projection area of the cortex in response to repetitive (one every five seconds) light flashes. During the experiments the rabbits were semi-restricted in their movements by being wrapped in burlap sacking, but were otherwise unrestrained by either mechanical devices or drugs. We confirmed Purpura's observations that LSD-25 (25-50 ttg total dose) increased somewhat the cortical evoked response (figure 5, A).
More significant, however, was an additional observation pertaining to variability of response. It is common knowledge that under physiological conditions and particularly in awake animals, indicators such as evoked responses vary greatly in amplitude when the stimulus is given repetitively to produce a whole series of evoked signals. In our experimental situation, the evoked responses (to constant, standardized stimuli) changed in amplitude over a range as great as 1:20 or more. LSD-25, in the dose mentioned, drastically decreased this variability down to about 25 per cent of the original value (figure 5, VA, and figure 6). The latency of the response (i.e., the time elapsed between the stimulus and the appearance of the response, signaling the transmission time from the retinal light receptors to the cortex) was somewhat decreased, and again its variability was greatly reduced under LSD-25 (figure 5, VL).
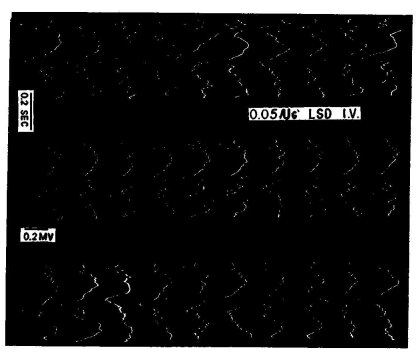
Figure 6. Ten consecutive responses to repetitive light flashes are recorded in the visual projection area of a rabbit. From such records the curves shown in figure 5 were constructed. Note the complex response, consisting of primary response (at about zo milliseconds after stimulus, at extreme left of records) and series of later responses with large component about zoo milliseconds after stimulus. Left column before, middle column one minute after, and right column one hour after, LSD-25. Note marked decrease in variability of primary response and of later responses after LSD-25. (From Koella and Wells, op. cit.)
One may assume that the response variability is the manifestation of a spontaneous endogenous fluctuation in excitability of the various synaptic sites interposed in the afferent sensory pathways. One may go further by postulating that this variability is of importance for the well-being of the organism and that the psychotomimetic action of substances like LSD-25 is, in part at least, due to their ability to decrease this variability. In this connection, it is noteworthy to mention that Bergen and collaborators (1962), in rabbit preparations similar to those described above, demonstrated a variability-reducing effect, similar to that of LSD-25, as a result of administering plasma protein fractions from schizophrenic patients. Fractions from normal controls did not have such an effect.
Related to the findings of Koella and Wells are the observations of Goldstein and his co-workers (1963). These investigators have shown by means of automatic analyzing techniques that the alpha-wave output in the EEG varies in time. When they recorded the EEG over extended periods, they found that the number and amplitude of the alpha waves tended to increase and decrease in what was probably a random fashion. Goldstein and his colleagues demonstrated that this variability was reduced by LSD-25 and that it was also a priori smaller in schizophrenic patients as compared with normal control subjects.
Perhaps also related to these observations on variability of response and its reaction to LSD-25 are the findings of Witt (1951). This author, in his extended studies on the web-building ability of spiders, found that LSD-25 in low doses (less than o.o5 jig per animal) led to increased regularity of the web angles.
These few examples taken from the work of neuropharmacologists have shown that LSD-25 has a number of pronounced effects on the central nervous system. They also offered an opportunity to acquaint the reader with some of the techniques (though by no means all) used by the neuropharmacologist to study the effects of drugs on the nervous system. In some cases, it seems possible to relate the effects of LSD-25 to the behavioral and psychic actions of this drug. In other instances, we are still far from establishing such functional relations. It is hoped that renewed efforts with new and better techniques will enable us in the not-too-distant future to explain the whole "experience" produced by these substances, the whole "model psychosis," in terms of neural events. Should this be the case, one also would be a giant step closer to establishing a functional pathogenesis of endogenous mental disease; i.e., one would be able to explain some or all the behavioral symptoms of the mentally ill in terms of abnormal neuronal function. It seems that with such a prospect in mind one could not think of a nobler task for a 'substance like LSD-25.
| < Prev | Next > |
|---|












