CHAPTER II THE HEALTH AND PSYCHOLOGICAL EFFECTS OF CANNABIS USE
| Reports - The Global Cannabis Commission Report |
Drug Abuse
CHAPTER II THE HEALTH AND PSYCHOLOGICAL EFFECTS OF CANNABIS USE
INTRODUCTION
Any proposal to change the legal status of cannabis must take into account the health and psychological effects of its use. In modern societies, a finding of adverse effects does not settle the issue of the legal status of a commodity; if it did, alcohol, automobiles and stairways, for instance, would all be prohibited, since use of each of these results in substantial casualties. Instead, the scope and extent of the adverse effects become one of the considerations to be taken into account in the policy decisions. The international drug control treaties, and most national drug control laws, divide different psychoactive substances between different “schedules”, with different levels of control and different penalties for trafficking and use, that are supposed to be matched, among other things, to the drug’s potential for adverse consequences.
The health and psychological effects of regular cannabis use are not as well understood as those of alcohol and tobacco, but epidemiological research over the past decade has provided evidence that it can have adverse effects on some users, particularly those who initiate use in adolescence and use more than weekly for years during young adulthood. In the decade since the health effects of cannabis were last reviewed by the World Health Organization (Kalant et al., 1999; WHO Programme on Substance Abuse, 1997), there has been a substantial increase in epidemiological and clinical research on the consequences of cannabis use by adolescents and young adults (see Castle & Murray, 2004; Grotenhermen, 2007; Hall & Pacula, 2003; Kalant, 2004; Roffman & Stephens, 2006 for recent reviews).
This chapter summarises the most probable adverse health and psychological effects of acute and chronic cannabis use. It focuses on those effects that are of greatest potential public health significance, as indicated by their likelihood of affecting a substantial proportion of cannabis users. The adverse effects considered include: the effects of cannabis use on the risk of motor vehicle crashes, a cannabis dependence syndrome, the effects of cannabis smoking on the respiratory and cardiovascular systems, the effects of regular cannabis use on adolescent psychosocial development and mental health, and the effects of chronic cannabis use on cognitive performance and brain function. Priority is given to evidence from well-controlled human epidemiological studies and clinical and laboratory studies of the effects of acute and chronic cannabis use.
At the end of the chapter we consider the evidence in a comparative framework: what can be said about the relative adverse impact on public health of cannabis use compared to use of other psychoactive substances, licit and illicit? As noted, such a comparative perspective is needed for policy decisions about the legal status of cannabis.
ACUTE HEALTH EFFECTS OF CANNABIS
Cannabis produces euphoria and relaxation, alters perception, distorts time, and intensifies ordinary sensory experiences, such as, eating, watching films, appreciating nature, and listening to music. Users’ short-term memory and attention, motor skills, reaction time and skilled activities are impaired while they are intoxicated (Hall & Pacula, 2003; Iversen, 2007). These effects develop rapidly after smoking cannabis and typically last for 1 to 2 hours (Iversen, 2007). Their onset is delayed for 1 to 4 hours after oral use (Iversen, 2007).
Cannabis users are typically seeking one or more of these effects when they use. But use can also result in unsought and adverse effects. The most common unpleasant effects of acute cannabis use are anxiety and panic reactions (Hall & Pacula, 2003; Kalant, 2004). These may be reported by naive users and they are a common reason for discontinuing use. More experienced users may also report these effects after receiving a much larger than usual dose of THC (Hall & Pacula, 2003). Recent research suggests that CBD can moderate the psychotogenic effects of THC (Morgan & Curran, 2008), but it remains to be tested whether cannabis products with lower THC:CBD ratios also produce fewer anxiety and panic reactions.
THC appears to produce its effects by acting on specific cannabinoid (CB1 and CB2) receptors on the surfaces of cells (Pertwee, 2008). The CB1 receptor is widely distributed in brain regions that are involved in cognition, memory, reward, pain perception and motor coordination (Iversen, 2007; Murray et al., 2007). These receptors also respond to a naturally-occurring (or endogenous) cannabinoid ligand, anandamide, which produces similar effects to THC but is less potent and has a shorter duration of action (Pertwee, 2008). Neuroimaging studies of the acute effects of cannabis in humans using positron emission tomography (PET) methods confirm findings in animals that THC increases activity in the frontal and paralimbic regions of the brain and in the cerebellum (Chang & Chronicle, 2007).
Acute Toxicity And Fatal Overdose
The acute toxicity of cannabinoids is very low by comparison with other psychoactive drugs, because they do not depress respiration like the opioids, or have toxic effects on the heart and circulatory system like cocaine and other stimulants (Gable, 2004; Kalant, 2004). There have been two reported human deaths from cannabis poisoning in the world medical literature (Gable, 2004), but it is not clear that THC was responsible for these deaths (Kalant, 2004). The dose of THC required to produce 50% mortality in rodents is extremely high by comparison with other commonly used drugs; the estimated fatal human dose is in the range of 15 (Gable, 2004) to 70 g (Iversen, 2007), many times greater than the dose that even heavy users could consume in a day (Gable, 2004).
Cannabis increases heart rate and produces complex changes in blood pressure (Chesher & Hall, 1999). There have been reported deaths from myocardial infarction after cannabis use in young adults (e.g. Bachs & Morland, 2001), but these have been rare and they may have occurred in persons with pre-existing, undiagnosed heart disease (Kalant, 2004; and see below).
Accidental Injury
The greatest public health concern about the acute effects of cannabis is that intoxicated drivers may cause motor vehicle crashes (Hall & Pacula, 2003). In laboratory studies, cannabis produces dose-related decrements in cognitive and behavioural performance that may affect driving (Ramaekers et al., 2004; Robbe,1994). Specifically, it slows reaction time and information processing, and impairs perceptual-motor coordination, motor performance, short term memory, attention, signal detection, and tracking behaviour (Ramaekers et al., 2004; Solowij, 1998). These effects increase with THC dose, and are larger and more persistent in tasks requiring sustained attention (Solowij, 1998).
Surveys find that drivers who report using cannabis are twice as likely to report being involved in accidents as drivers who do not (e.g. Asbridge et al., 2005; Hingson et al., 1982 b). It has been difficult to decide how much of the relationship reflects the effects of cannabis on accident risk, the effects of concurrent alcohol use, and the risk behaviour of heavier cannabis users. One recent study found that the association disappeared after controlling for these factors (Fergusson & Horwood, 2001), while another (Blows et al., 2005) found that “habitual” cannabis users had a nine-fold higher crash risk that persisted after controlling for confounding factors including blood-alcohol concentration (BAC).
Studies of the effects of cannabis upon on-road driving performance have reported more modest impairments than comparable doses of alcohol (Smiley, 1999). This appears to be because cannabis-intoxicated drivers drive more slowly and take fewer risks than alcohol-intoxicated drivers (Smiley, 1999). More recent studies of the effects of cannabis on driving performance on the road that have used doses closer to typical recreational doses (Robbe, 1994) have found small but consistent decrements in driving performance.
Cannabis is the illicit drug most often detected in the bodily fluids of drivers who have been injured or killed in motor vehicle crashes (see Kelly et al., 2004 for a review). It has been uncertain for a number of reasons whether cannabis has played a causal role in these accidents (Hall et al., 2001). Firstly, earlier studies measured inactive cannabinoid metabolites in blood and urine, which only indicated that cannabis had been used within the past few days; they did not establish that the driver was intoxicated at the time of the accident (see Bates & Blakely, 1999; Hall et al., 2001; Kelly et al., 2004 for reviews). Secondly, many drivers with cannabinoids in their blood also had high blood alcohol levels (Bates & Blakely, 1999; Hall et al., 2001).
Better-controlled epidemiological studies have recently provided better evidence that cannabis users who drive while intoxicated are at increased risk of motor vehicle crashes. Gerberich et al. (2003) found that current cannabis users had higher rates of hospitalisation for injury from all causes than former cannabis users or non-users in a cohort of 64,657 patients from a Health Maintenance Organization. The relationship for motor vehicle accidents (relative risk (RR) = 1.96) persisted after statistical adjustment among men but not among women. Women in the cohort also had much lower rates of cannabis use and accidents. Mura et al. (2003) found a similar relationship in a study of THC in the serum of 900 persons hospitalised for motor vehicle injuries and 900 age-and-sex matched controls in France. They did not, however, statistically adjust for blood-alcohol level which was found in 40% of cases with THC present.
Drummer et al. (2004) assessed THC levels in blood in 1420 Australian drivers killed in accidents. They found cannabis users were more likely to be culpable for accidents (odds ratio (OR) = 2.5) and there was a higher accident risk (OR = 6.6 [95% CI: 1.5, 28.0]) among those with THC levels greater than 5 nanograms per millilitre. Their findings differed from those of another Australian study (Longo et al., 2000) that did not find a relationship between THC and culpability. However, this study involved injuries rather than fatalities, there were longer delays between these accidents and drug testing, and the average levels of THC detected in blood were much lower than those reported by Drummer et al. (2004).
Laumon et al. (2005) compared blood THC levels in 6,766 culpable and 3,006 nonculpable drivers in France between October 2001 and September 2003. There was an increased culpability for drivers with THC detected in their blood at levels of greater than 1 ng/ml (OR = 2.87) compared to a 15.5 increase for drivers with BAC greater than 0.05 g/l. There was a dose-response relationship between THC and culpability that persisted after controlling for BAC, age and time of accident. On these data, 2.5% of fatal accidents in France could be attributed to cannabis and 29% to alcohol (with a BAC of greater than 0.05%).
Bedard et al. (2007) examined the relationship between cannabis use and accident risk in 32,543 drivers killed in the USA between 1993 and 2003. They found a dose response relationship between BAC and culpability and a more modest association (OR = 1.39 [99% CI: 1.21-1.59]) between culpability and cannabis use assessed in a variety of ways, including inactive metabolites. The association was attenuated but still significant after adjustment for crash history, age, convictions for drink driving and BAC (OR = 1.29).
A convergence of fallible evidence thus suggests that cannabis use increases the risk of motor vehicle crashes 2-3 times (Ramaekers et al., 2004). The size of the effect on driving risks is much more modest than that of alcohol (with ORs for cannabis ranging from 1.3-3, compared with 6-15 for alcohol). The relationship may be attenuated because impairment is not as directly related to blood THC levels as is BAC. The estimated contribution of cannabis use to accident deaths has been much smaller than that of alcohol (2.5% vs. 29%). This probably reflects a combination of the lower crash risks in cannabis-impaired drivers and the lower prevalence of cannabis-impaired drivers.
The motor vehicle crash risks of cannabis use are of public health significance because of the high rates of cannabis use among young adults at highest risk of injury and death from car crashes. An additional concern is that the combined use of cannabis and alcohol (which is in some countries more common that cannabis use alone) probably increases the crash risk over that of either drug used on its own (Ramaekers et al., 2004). The policy challenge is to define a level of THC in blood that can be used by courts to define impairment (Grotenhermen et al., 2007).
Immunological Effects
Cannabinoid CB2 receptors are found in the immune system, (Roth et al., 2002), and animal studies suggest that high doses of cannabis extracts and of THC impair immune functioning. A number of studies in mice and guinea pigs suggest that high doses (200 mg/kg) of cannabinoids decrease resistance to infection with Lysteria monocytogenes (Morahan et al., 1979) and herpes simplex type 2 virus (e.g. Cabral & Pettit, 1998). There have, however, been very few epidemiological studies of immune system functioning and disease susceptibility in heavy cannabis users to assess how serious these immunological risks may be (Cabral & Pettit, 1998; Klein et al., 2001; Roth et al., 2002).
Several epidemiological studies have examined the effects of self-reported cannabis use on progression to AIDS among HIV positive homosexual men. Kaslow et al. (1989) reported a prospective study of progression to AIDS among 4954 HIV-positive homosexual and bisexual men. Cannabis use did not predict increased progression to AIDS, and it was not related to changes in immunological functioning. There was also no relationship between marijuana use and progression to AIDS in HIV-seropositive men in the San Francisco Men's Health Study (N=451) over 6 years (DiFranco et al., 1996). There was an increased risk of progression to AIDS among cannabis users in the Sydney AIDS Project, but the Institute of Medicine (1999) has described this finding as "less reliable" than those of Kaslow et al. and DiFranco et al. because the study had a short follow-up period, and many of the “HIV-positive cases” already had AIDS. A study of mortality among a cohort enrolled in a health insurance plan (Sidney et al., 1997 a) did find an association between cannabis use and death from AIDS, but this was attributed to confounding of cannabis use and sexual preference (which was not assessed in the study).
REPRODUCTIVE EFFECTS OF CANNABIS USE
Cannabis is widely used by adolescents and young adults during the peak age for reproduction. Animal studies in the mid-1970s raised concerns that cannabis use during this period could adversely affect reproductive outcomes because large doses of THC reduced the secretion of gonadal hormones in both sexes (Brown & Dobs, 2002) and adversely affected foetal development (Bloch, 1983).
Effects on the male and female reproductive systems
In animals, marijuana, crude marijuana extracts, THC and some purified cannabinoids depress male reproductive endocrine function (Bloch, 1983; Brown & Dobs, 2002). If used chronically, cannabis may reduce plasma testosterone, retard sperm maturation, reduce sperm count and sperm motility, and increase the rate of abnormal sperm production (Bloch, 1983; Murphy, 1999). The mechanisms for these effects are unclear but probably reflect the effects of THC on the testes, and indirectly on the hypothalamic hormones that stimulate the testes to produce testosterone (Brown & Dobs, 2002).
Studies of the effects of cannabis on human male reproductive function have produced mixed results (Brown & Dobs, 2002). An early study that reported reduced testosterone, sperm production, and sperm motility and increased abnormalities in sperm (Kolodny et al., 1974) was not replicated in later studies (Brown & Dobs, 2002). The latter included a larger, well-controlled study of the effects of three weeks of daily cannabis use on plasma testosterone (Mendelson et al., 1974). Other studies have produced both positive and negative evidence of an effect of cannabinoids on testosterone for reasons that are not well understood (Brown & Dobs, 2002). If there are effects of cannabis on male reproductive functioning, their clinical significance in humans is uncertain because testosterone levels have generally been within the normal range in studies that found effects (Hollister, 1986). A recent study of outcomes of in-vitro fertilisation (IVF) and gamete intrafallopian transfer (GIFT) reported that males who reported using cannabis regularly fathered children with lower birth weights (Klonoff-Cohen et al., 2006), although the mechanism for such an effect is unclear.
Animal studies also suggest that cannabis extracts and pure THC interfered with the hypothalamic-pituitary-gonadal axis in female rats (Bloch, 1983; Brown & Dobs, 2002), while chronic exposure delayed oestrous and ovulation (Murphy, 1999). There have been very few experimental studies of the effects of cannabis on the human female reproductive system, because of fears that cannabis use may produce birth defects in women of childbearing age. Mendelson and Mello (1984) observed hormonal levels in a group of female cannabis users (all of whom had undergone a tubal ligation) and failed to find any evidence that chronic cannabis use affected sex hormones or the duration of the cycle. A more recent observational study of the outcomes from IVF and GIFT found that women who had a history of past regular cannabis use had fewer oocytes retreived and fertilised than those who had not smoked cannabis (Klonoff-Cohen et al., 2006).
Foetal development and birth defects
In animal studies very high doses of cannabis can produce resorption, growth retardation, and malformations in mice, rats, rabbits, and hamsters. (Bloch, 1983). Birth malformations have been observed more often after crude marijuana extract rather than THC, suggesting that other cannabinoids may have teratogenic effects. Bloch (1983) concluded that THC was unlikely to be teratogenic in humans because “the few reports of teratogenicity in rodents and rabbits indicate that cannabinoids are, at most, weakly teratogenic in these species” (p 416).
Epidemiological studies of the effects of cannabis use on human development have produced mixed results for a number of reasons. Firstly, heavy cannabis use is relatively rare during pregnancy, so very large sample sizes are needed to detect any adverse effects on foetal development (Fried & Smith, 2001). Many of the studies have been too small to detect such effects. Secondly, the stigma of admitting to drugs use during pregnancy encourages under-reporting (Day et al., 1985). If a substantial proportion of cannabis users are misclassified as non-users, any relationship between cannabis use and adverse outcomes will be attenuated. Thirdly, there are difficulties in interpreting associations that have been reported between adverse pregnancy outcomes and cannabis use (e.g. Forrester & Merz, 2007) because cannabis users are also more likely to use tobacco, alcohol and other illicit drugs during pregnancy (Eyler & Behnke, 1999), and they are also less likely to seek antenatal care and more likely to have poorer nutrition than women who do not use cannabis (Tennes et al., 1985).
Cannabis use in pregnancy is more consistently associated with reduced birth weight (Fergusson et al., 2002a; Gibson et al., 1983; Hatch & Bracken, 1986; Zuckerman et al., 1989). This relationship was found in one of the largest, best-controlled studies (e.g. Fergusson et al., 2002a), where it persisted after statistically controlling for confounding variables (e.g. Fergusson et al., 2002a; Hatch & Bracken, 1986; Zuckerman et al., 1989) A meta-analysis of these studies (English et al., 1997) found that regular cannabis-smoking during pregnancy reduced birth weight, though less than tobacco-smoking.
There has been no consistent relationship between cannabis use and birth abnormalities. Early case reports of birth abnormalities in children born to women who had smoked cannabis during pregnancy have generally not been supported by epidemiological studies (Gibson et al., 1983; Hingson et al., 1982a; Tennes et al., 1985; Zuckerman et al., 1989). One recent study found associations between cannabis use during pregnancy and a large number of birth defects in infants born in Hawaii between 1986 and 2002 (Forrester & Merz, 2007), but the study was unable to control for important confounding variables. Zuckerman et al. (1989) report failing to find an increased risk of birth defects. This was a convincing negative finding because they studied a large sample of women among whom there was a substantial rate of cannabis use that was verified by urinalysis. There was a low rate of birth abnormalities among the cannabis users, and no suggestion of an increase by comparison with the controls.
Post-natal effects of intrauterine exposure to cannabinoids
The Ontario Prospective Prenatal Study has studied developmental and behavioural abnormalities in children born to women who reported using cannabis during pregnancy (Fried & Smith, 2001; Fried & Watkinson, 2000; Hutchings & Fried, 1999). In this study, mothers were asked about their drug use during pregnancy and their children were measured on the Brazelton scales after birth, neurologically assessed at one month, and again at six and twelve months.
There was some developmental delay shortly after birth in the visual system, and increased tremor and startle among the children of cannabis users (Fried & Smith, 2001), but these behavioural effects faded by one month, and no differences were detected on ability tests at six and twelve months. Subtle behavioural effects of cannabis were subsequently reported at 36 and 48 months, but not at 60 and 72 months (Fried & Smith, 2001). These results are suggestive of a subtle developmental impairment occurring among children who had experienced a shorter gestation and prematurity (Fried & Smith, 2001). The cohort has now been followed up to age 9 to 12 years. No differences were found between children who were and were not exposed to cannabis during pregnancy on full scale IQ scores, but there were small differences in measures of perceptual organisation and higher cognitive processes (Fried & Smith, 2001).
Attempts to replicate these OPPS findings have been mixed. Tennes et al. (1985) studied the relationship between cannabis use during pregnancy and postnatal development in 756 women, a third of whom reported cannabis use during pregnancy.
They found no evidence of impaired development of the visual system, no increased risk of tremor or startle at birth, and no differences at one year between the children of users and nonusers. Day et al. (1994), by contrast, followed up children at age three who were born to 655 teenage women in Pittsburgh between 1990 and 1995. They found poorer performances on memory and verbal scales of the Stanford-Binet Intelligence Scale at age 3 in children of women who reported cannabis use during pregnancy. At age 6, prenatal cannabis exposure was associated with reduced height, after controlling for alcohol and tobacco use and other predictors of impaired growth (Cornelius et al., 2002). By age 10, antenatal cannabis exposure was associated with increased delinquency and problem behaviour (Goldschmidt et al., 2000).
Overall, the post-natal behavioural effects of prenatal cannabis exposure appear to be modest (Huizink & Mulder, 2006). Their existence remains uncertain because of the small size of the effects and their tendency to come and go at different ages. The causal interpretation of the effects reported is complicated by the inability of these studies adequately to control for confounding variables (Huizink & Mulder, 2006), such as other drug use during pregnancy, poor parenting skills, and shared genetic risks for impaired cognitive functioning in both mothers and their infants.
Maternal cannabis use and childhood cancers
Cannabis smoking has also been linked with cancers among children born to mothers who used cannabis during pregnancy in three case-control studies. In none was there an a priori reason to expect a relationship between cannabis use and these cancers.
An association between maternal cannabis use and childhood cancer was reported in a case-control study of Acute Nonlymphoblastic Leukemia (ANLL) (Neglia et al., 1991; Robinson et al., 1989). Maternal cannabis use was assessed as a potential confounder. Mothers of cases were 11 times more likely to have used cannabis before and during their pregnancy than were the mothers of controls. The relationship persisted after statistical adjustment for other risk factors. Reporting bias was an alternative explanation because the reports of cannabis use were obtained after diagnosis of ANLL and the rate of cannabis use was lower in controls than in general population surveys. Two other case-control studies have reported an increased risk of rhabdomyosarcoma (Grufferman et al., 1993) and astrocytomas (Kuijten et al., 1992) in children born to women who reported using cannabis during their pregnancies In each study, cannabis use was one of a large number of confounding variables that were measured. The possibility of measurement bias in these studies was quite high (Hashibe et al., 2005).
There have been no increases in the incidence of these childhood cancers over the period 1979-1995 that could be explained by increased maternal cannabis use during pregnancy (Reis et al., 2000). The incidence of ANLL, for example, remained steady between 1979 and 1995 (Smith et al., 2000) despite the very high relative risk reported for this cancer. The same was true for soft-tissue sarcomas (which include rhabdomyosarcomas) (Gurney et al., 2000b). Central nervous system (CNS) malignancies (about 52% of which are astrocytomas) increased in incidence between 1979 and 1995 (Gurney et al., 2000a) but in a way that was unlikely to reflect maternal cannabis use. Incidence was steady between 1979 and 1985, when it abruptly increased, and it remained steady thereafter (Gurney et al., 2000a). Magnetic resonance imaging (MRI) became widely used in the USA in 1985, which suggests that the increase was an artefact of improved diagnosis rather than an increase in incidence (Gurney et al., 2000a).
THE HEALTH EFFECTS OF CHRONIC CANNABIS USE
“Chronic” cannabis use is a broad term meant to cover the regular (especially daily or near daily) use over periods of years. Epidemiological studies of relationships between chronic cannabis use and various human diseases are now being conducted. The major problems with these studies are assessing exposure to cannabis over extended periods and excluding alternative explanations of the associations. A major problem in interpreting epidemiological studies is that cannabis use is correlated with other drug use which is known to adversely affect health (e.g. alcohol and tobacco use). Generally, the heavier the cannabis use, the more likely it is that the person uses other licit (alcohol and tobacco) and illicit drugs (amphetamines, hallucinogens, cocaine, and heroin). This makes it difficult confidently to attribute some of the adverse health effects found in cannabis users to their cannabis use (Hall, 1999). Statistical control of confounding variables is the best available approach to deal with this problem.
In the following sections we discuss the evidence on the adverse health and psychological effects that have been most commonly attributed to regular cannabis use. We begin with the question of whether cannabis is a drug of dependence, and then consider the most plausible adverse physical health effects of chronic use, namely respiratory and cardiovascular disease. We end by exploring the evidence on the adverse psychological effects that chronic cannabis use may have on adolescent development and the mental health of young adults via psychosis, depressive disorders and cognitive impairment.
Cannabis Dependence
Both the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) and the International Classification of Diseases (ICD-10) include a diagnosis of cannabis dependence that is characterised by marked distress resulting from a recurring cluster of problems that reflect impaired control over cannabis use and continued cannabis use despite harms arising from its use. Community mental health surveys indicate that in many developed societies cannabis dependence is the most common type of drug dependence after alcohol and tobacco (Anthony & Helzer, 1991; Hall et al., 1999b; Kessler et al., 1994; Stinson et al., 2006). About 2% of adults meet criteria for cannabis abuse and dependence in the past year (Stinson et al., 2006; Swift et al., 2001), with a lifetime rate of 4%-8% in US adults (Anthony & Helzer, 1991; Stinson et al., 2006). The risk of dependence is around 9% among persons who have ever used cannabis (Anthony et al., 1994; Swift et al., 2001) and around one in six for young people who initiate in adolescence (Swift et al., 2001). These risks compare with risks of 32% for nicotine, 23% for heroin, 17% for cocaine, 15% for alcohol and 11% for stimulant users (Anthony et al., 1994). Those at highest risk of cannabis dependence have a history of poor academic achievement, deviant behaviour in childhood and adolescence, nonconformity and rebelliousness, poor parental relationships, and a parental history of drug and alcohol problems (Coffey et al., 2003; Swift et al., 2001).
Animals and humans develop tolerance to many of the behavioural and physiological effects of THC (Lichtman & Martin, 2005; Maldonado, 2002). The cannabinoid antagonist SR 141716A precipitates a withdrawal syndrome in rats, mice and dogs (e.g. Selley et al., 2003) that is reversed by administering THC (Lichtman et al., 2001). Down-regulation of CB1 receptors may underlie the development of tolerance (Lichtman & Martin, 2005).
Similar withdrawal symptoms occur in humans (Budney & Hughes, 2006). This includes subjects abruptly withdrawn after 30 days on high dose THC (Jones et al., 1976), and chronic recreational cannabis users (Kouri & Pope, 2000), including such users who were not seeking help to stop use (Budney et al., 2001). Long-term users seeking help to stop often report withdrawal symptoms that include anxiety, insomnia, appetite disturbance and depression (Budney & Hughes, 2006; Budney et al., 2004), and they also report using cannabis to relieve withdrawal symptoms (Budney et al., 2007)
Over the past two decades, increasing numbers of cannabis users have sought help in the USA, Europe, and Australia because of difficulties they experienced in stopping their cannabis use (AIHW, 2006; EMCDDA, 2003; SAMHSA, 2004). Some have argued that this increase is the result of increased diversion of cannabis users into treatment by the courts (Zimmer & Morgan, 1997). This seems less likely to wholly explain similar increases in the Netherlands, where the use of cannabis is de-facto largely decriminalised (Dutch National Alcohol and Drug Information System, 2006).
Cannabis dependence can be treated on an outpatient basis using cognitive-behavioural therapy (CBT) (McRae et al., 2003). CBT reduces cannabis use and cannabis-related problems (Denis et al., 2006; McRae et al., 2003), although the proportion of those who achieve enduring abstinence is modest (Denis et al., 2006). Continuous abstinence rates have been as low as 15% 6-12 months after treatment (Copeland et al., 2001; Denis et al., 2006), with the best abstinence rate (35%) reported from combined CBT and contingency management using vouchers in a sample of 20 patients (McRae et al., 2003). Pharmacological efforts to improve the management of cannabis withdrawal symptoms (Kleber et al., 2007) have not to date found any agent superior to placebo (Budney & Hughes, 2006; Hart, 2005).
The Respiratory Risks Of Cannabis Smoking
Over the past two decades, cross-sectional and longitudinal studies in the USA (Tashkin et al., 2002) and New Zealand (Aldington et al., 2007; Taylor et al., 2002; Taylor et al., 2000), have shown that people who are regular smokers of cannabis have more symptoms of chronic bronchitis (wheeze, sputum production and chronic coughs) than non-smokers (see Tashkin et al., 2002; Tetrault et al., 2007 for reviews). The immunological competence of the respiratory system in people who only smoke cannabis is also impaired, increasing their susceptibility to respiratory infections and pneumonia, and their use of health services for these infections (Tashkin et al., 2002).
The effects of long term cannabis smoking on respiratory function are less clear (Tashkin et al., 2002; Tetrault et al., 2007). A longitudinal study (Taylor et al., 2002; Taylor et al., 2000) of respiratory function in 1037 New Zealand youths followed from birth until the age of 21 (Taylor et al., 2000) and 26 (Taylor et al., 2002) found that cannabis-dependent subjects had impaired respiratory function. But this finding has not been replicated in a longer follow-up study of Tashkin’s cohort (Tashkin et al., 2002).
There is no evidence to date that chronic cannabis smoking increases the risk of emphysema (Tashkin, 2005). Follow up studies of Tashkin’s cohort after 8 years failed to find increased rates of emphysema in marijuana-only smokers (Tashkin, 2005). The same result has recently been reported in a similarly-recruited group of heavy cannabis-only smokers in New Zealand (Aldington et al., 2007).
Respiratory Cancers
There are good reasons for believing that cannabis can cause cancers of the lung and the aerodigestive tract (Hall & MacPhee, 2002; Hashibe et al., 2005). Cannabis smoke contains many of the same carcinogens as tobacco smoke, which is a cause of respiratory cancer (Hashibe et al., 2005; Marselos & Karamanakos, 1999).
Some of these carcinogens occur at higher levels in cannabis than tobacco smoke (Moir et al., 2008). Cannabis smoke is mutagenic in the Ames test and causes cancers in the mouse skin test (MacPhee, 1999; Marselos & Karamanakos, 1999). Cannabis smokers inhale more deeply than tobacco smokers, retaining more tar and particulate matter (Hashibe et al., 2005; Tashkin, 1999); and chronic cannabis smokers show many of the pathological changes in lung cells that precede the development of cancer in tobacco smokers (Tashkin, 1999).
The results of epidemiological studies of upper respiratory tract cancers in cannabis users have been mixed. Sidney et al. (1997b) studied cancer incidence in an 8.6 year follow up of 64,855 members of the Kaiser Permanente Medical Care Program. There was no increased risk of respiratory cancer at follow-up among those who had ever used cannabis and current cannabis users. Males who had smoked cannabis had an increased risk of prostate cancer (RR = 3.1), and so did current cannabis smokers (RR = 4.7).
Zhang et al. (1999), by contrast, found an increased risk of squamous cell carcinoma of the head and neck among cannabis users in a case-control study of 173 persons with this cancer and 176 controls (blood donors matched on age and sex from the same hospital). There was an odds ratio of 2 for cannabis smoking after adjusting for cigarette smoking, alcohol use, and other risk factors. Two other case-control studies of oral squamous cell carcinoma, however, have failed to find any association between cannabis use and oral cancers. Llewellyn et al. (2004) failed to find any association between self-reported cannabis use and oral cancers in a study of 116 cases (identified from a cancer register) and 207 age- and sex-matched controls (sampled from the same general practices as the cases). Rosenblatt et al. also reported a null finding in a community-based study of 407 cases and 615 controls aged 18 to 65 years in Washington State (Rosenblatt et al., 2004).
Case-control studies of cannabis smoking and lung cancer have produced more consistent evidence of harm (Mehra et al., 2006). A Tunisian case control study of 110 cases of hospital-diagnosed lung cancer and 110 community controls found an association with cannabis use (OR= 8.2) that persisted after adjustment for cigarette smoking, water pipe and snuff use. A Moroccan case-control study of 118 cases and 235 control subjects also found an increased risk of lung cancer (OR = 5.6) among users those who smoked a combination of cannabis flowers and tobacco but a more marginal relationship for those who only smoked cannabis. A New Zealand case-control study of lung cancer in 79 adults under the age of 55 years and 324 community controls (Aldington et al., 2008) reported a dose-response relationship between lung cancer risks and frequency of cannabis use. Among the highest third of cannabis users there was a 5.7 times higher risk of lung cancer.
Uncertainties remain about the risk of oral and respiratory cancers among cannabis smokers (Hashibe et al., 2005; Mehra et al., 2006). The risk of oral cancer is small compared to that for tobacco smoking, given the small relative risk in the only positive study (Rosenblatt et al., 2004). The findings from the case-control studies of lung cancer are more suggestive of higher risks, but the measures of cannabis use in these studies have been relatively crude and it is unclear how well these studies have been able to control for tobacco smoking. Larger cohort studies and better-designed case-control studies of tobacco-related cancers are needed to clarify the relationship between cannabis smoking and the risks of these cancers (Hall & MacPhee, 2002; Mehra et al., 2006).
Cardiovascular Effects Of Cannabis Smoking
In humans and animals, cannabis and THC produce dose-related increases in heart rate (Chesher & Hall, 1999; Jones, 2002). The hearts of healthy young adults are only mildly stressed by this effect and tolerance develops quickly (Institute of Medicine, 1999; Jones, 2002; Sidney, 2002). There are more reasons for concern about these effects in older adults, who are at increased risk of ischaemic heart disease, hypertension, and cerebrovascular disease (Jones, 2002; Sidney, 2002). There are case reports of myocardial infarction, arrythmias and vascular complications in young otherwise healthy cannabis users (Aryana & Williams, 2007), but there have been few epidemiological or other controlled studies.
A case-crossover study by Mittleman et al. (2001) of 3882 patients who had had a myocardial infarction suggested that cannabis use increased the risk of a myocardial infarction 4.8 times in the hour after use (compared with 24 fold for cocaine). Mittleman et al. estimated that a 44-year-old adult who used cannabis daily would increase his or her annual risk of an acute cardiovascular event by 1.5% to 3%. Mukamal et al (2008) recently provided support for Mittleman et al. in a prospective study of 1913 adults hospitalized for myocardial infarction. They found a dose-response relationship between self-reported cannabis use at baseline and mortality over the subsequent 3.8 years. The mortality risk increased 2.5 times for less-than-weekly users, and 4.2 times for more-than-weekly users. Both sets of findings are supported by double-blind laboratory studies which show that smoking cannabis produces symptoms of angina in patients with heart disease (Aronow & Cassidy, 1974; 1975; Gottschalk et al., 1977).
CHRONIC CANNABIS USE AND BRAIN FUNCTION
Cognitive functioning
Cognitive impairment, particularly in short-term memory, is often reported by cannabis-dependent persons seeking help to stop using cannabis (Solowij, 1998). Controlled studies have not found that long-term use produces severe impairment of cognitive function (Solowij, 1998). Lyketsos et al. (1999) assessed cognitive decline on the Mini Mental State Examination (MMSE) in 1318 adults over 11.5 years. They found no relationship between cannabis use and decline in MMSE score, indicating that cannabis use did not produce gross cognitive impairment (Solowij, 1998). This study does not exclude the possibility of more subtle cognitive impairment, because the MMSE is a screening test that is not sensitive to small changes in cognitive functions, and in this study “heavy cannabis users” included anyone who ever reported smoking daily for more than 2 weeks.
There is evidence from controlled laboratory studies that long-term heavy cannabis users show more subtle types of cognitive impairment (Solowij, 1998). A major problem in interpreting these studies has been excluding the possibility that regular cannabis users had poorer cognitive functioning than controls before they started to use cannabis (Solowij, 1998). The better studies have matched users and non-users on estimated premorbid intellectual functioning (Solowij, 1998) or on cognitive test performance before the onset of cannabis use (e.g. Block et al., 2002).
These have found cognitive impairments associated with frequent and/or long term cannabis use (Block & Ghoneim, 1993; Block et al., 2002).
Solowij et al. (2002), for example, found few impairments in the neuropsychological performance of dependent, heavy cannabis users (near daily) with an average 10 years of regular use when compared to nonuser controls. Users with an average of 24 years of regular use, however, showed impaired attention and impaired verbal learning, retention, and retrieval. Solowij (1998) used event-related potentials to show impaired attention in shorter-term users (5+ years) and found that impairment increased with the number of years of cannabis use (Solowij, 1998; 2002).
Deficits in verbal learning, memory and attention are the most consistently replicated impairments in heavy cannabis users. There is disagreement about their explanation. They have been variously related to duration (Solowij et al., 2002), frequency (Pope et al., 2001) and cumulative dosage of THC (Bolla et al., 2002). The differential effects of frequency and duration of use and dose reported in some studies have not always been reported. Debate continues about whether these deficits are attributable to lingering acute drug effects, drug residues, abstinence effects, or gradual changes in the brain as a result of cumulative THC exposure (Pope et al., 1995; Solowij et al., 2002)
It is also uncertain whether cognitive functioning recovers after cessation of cannabis use. Solowij (1998) found partial recovery on a selective attention task after 2 years’ abstinence in a small group of ex-users, but brain event-related potential measures continued to show impaired information-processing, the severity of which was correlated with the years of cannabis use. Bolla et al. (2002) found persistent dose-related decrements in neurocognitive performance after 28 days of abstinence in heavy young users (mean age of 20 and 5 years use), while Pope et al. (2001) reported that memory impairments recovered after 28 days of abstinence. Another report on the latter sample (Pope et al., 2002) found persistent memory deficits in those who had started using before the age of 17.
Brain structure and function
An early finding of structural brain changes after prolonged cannabis use was not replicated (see Solowij, 1998; 1999 for reviews). One study used sophisticated measurement techniques to show that frequent but relatively short-term use of cannabis produced neither structural brain abnormalities nor global or regional changes in brain tissue volume or composition that are assessed by MRI (Block et al., 2000a). Other research has found reduced cortical grey matter and increased white matter in those who commenced using cannabis before age 17 compared to those who started using later (Wilson et al., 2000). It remains uncertain whether these findings reflect a cause or a consequence of early cannabis use.
A number of more recent studies have demonstrated altered brain function and metabolism in humans following acute and chronic use of cannabis using cerebral blood flow (CBF), positron emission tomography (PET), and electroencephalographic (EEG) techniques. Block and colleagues (2000b), for example, found that after 26 hours of supervised abstinence, regular cannabis users showed substantially lower resting brain blood flow than controls in the posterior cerebellum and prefrontal cortex. Similarly, Lundqvist, Jonsson and Warkentin (2001) showed lower mean hemispheric and frontal blood flow shortly after cessation of cannabis use. It remains to be determined whether these findings have any longer-term implications for cognitive functioning.
Loeber and Yurgelun-Todd (1999) have proposed that chronic cannabis use changes the cannabinoid receptors that act on the dopamine system, producing a reduction in brain metabolism in the frontal lobe and cerebellum. Recent studies using functional imaging techniques during cognitive tasks (e.g. Porrino et al., 2004; Quickfall & Crockford, 2006; Smith et al., 2004; Solowij et al., 2004) have shown diminished activity in the brains of chronic cannabis users compared to controls, even after cannabis users had abstained for 28 days (Block et al., 2002; Loeber & Yurgelun-Todd, 1999)
Changes in cannabinoid receptor activity in the hippocampus, prefrontal cortex and cerebellum have also been implicated in the cognitive impairments associated with chronic cannabis use. Yücel et al. (2008) recently reported reductions in the volumes of the hippocampus and amygdala in 15 long term users who had smoked 5 or more joints a day for 10 or more years. The size of the reductions was also inversely correlated with the duration of use. More functional brain imaging studies of this type on larger samples of long-term users hold the most promise in investigating whether the cognitive impairment found in long-term users is correlated with structural changes in areas of the brain that are implicated in memory and emotion and also richly endowed with cannabinoid receptors (Solowij et al., 2004).
THE CONSEQUENCES OF ADOLESCENT CANNABIS USE
Cannabis first began to be used by large numbers of young people in the USA in the early 1970s. In the subsequent 30 years, the proportion of young people who have used cannabis in many developed countries has increased, and age of first use has fallen in Australia, the USA and the Netherlands (Hall & Pacula, 2003). There has been considerable community concern about whether adolescent cannabis use increases poor educational outcomes (Lynskey & Hall, 2000), the use of heroin and cocaine (Fergusson et al., 2002b), and psychosis (Hall & Pacula, 2003).
Educational outcomes
Cannabis use acutely impairs memory and attention. Its regular use could potentially adversely affect learning in adolescents, producing poorer school performance and increasing early school drop out. Surveys typically find associations between cannabis use and poor educational attainment among school children and youth (e.g. Lifrak et al., 1997; Resnick et al., 1997; see Lynskey & Hall, 2000 for a review). Rates of cannabis use are also higher among young people who no longer attend school or who had high rates of absenteeism when at school (Fergusson et al., 1996; Lynskey et al., 1999).
One explanation of these associations is that cannabis use is a contributory cause of poor school performance (e.g. Kandel et al., 1986). A second possibility is that heavy cannabis use is a consequence of poor educational attainment (Hawkins et al., 1992). The first and second hypotheses could both be true if, for example, poor school performance increased cannabis use which in turn further impaired school performance. A third hypothesis is that cannabis use and poor educational attainment are the result of common factors that increase the risk of both early cannabis use and poor educational performance (Donovan & Jessor, 1985). This hypothesis is supported by the overlap between risk factors for early cannabis use and poor educational performance (see Hawkins et al., 1992 for reviews).
These explanations can only be distinguished by prospective studies of young people who are assessed over time on their cannabis use, educational attainment and potentially confounding factors, such as family and social circumstances, personality characteristics and delinquency (Lynskey & Hall, 2000). Such studies enable researchers to answer the question: do young people who use cannabis have poorer educational outcomes than those who do not, when we allow for the fact that cannabis users are more likely to have a history of poor school performance and other characteristics before they used cannabis?
Longitudinal studies (e.g. Fergusson et al., 1996) have typically found a relationship between cannabis use before the age of 15 years and early school leaving. This has persisted after statistical adjustment for differences between early cannabis users and their peers. (e.g. Duncan et al., 1998; Ellickson et al., 1998; Tanner et al., 1999). The most plausible hypothesis seems to be that the impaired educational performance in adolescent cannabis users is attributable to a higher pre-existing risk of these outcomes and a combination of the effects of acute intoxication upon cognitive performance, affiliation with peers who reject school, and a desire to make an early transition to adulthood (Lynskey & Hall, 2000). This hypothesis is supported by the lack of any relationship between marijuana use and dropping out of university in a longitudinal study of male American university students; among men who had used marijuana but no other drugs, “there was no evidence that drug use had any relation to dropping out that was independent of family background, relationships with parents in high school, and social values” (Mellinger et al., 1976).
Other illicit drug use
Surveys of adolescent drug use in the United States over the past 30 years have consistently shown three relationships between cannabis and the use of heroin and cocaine (see Kandel, 2002 for a review). First, almost all of those who tried cocaine and heroin first used alcohol, tobacco and cannabis. Second, regular cannabis users were most likely to later use heroin and cocaine. Third, the earlier the age at which cannabis was first used, the more likely a user was to use heroin and cocaine. These relationships have been confirmed in longitudinal studies of drug use in New Zealand (Fergusson & Horwood, 2000; McGee & Feehan, 1993).
Three types of explanation have been offered for these patterns of drug involvement. The first is that because cannabis and other illicit drugs are supplied by the same black market, cannabis users have more opportunities to use other illicit drugs than non-cannabis users (Cohen, 1976). The second hypothesis is that the association is that those who are early cannabis users are more likely to use other illicit drugs for reasons unrelated to their cannabis use (Morral et al., 2002). The third hypothesis is that the pharmacological effects of cannabis increase the propensity to use other illicit drugs (Murray et al., 2007).
Social environment and drug availability do play a role. Young people in the USA who have used alcohol or tobacco are more likely to report opportunities to use cannabis at an earlier age than those who have not (Wagner & Anthony, 2002). Moreover, those who had used cannabis reported more opportunities to use cocaine at an earlier age (Wagner & Anthony, 2002). In New Zealand, however, self-reported affiliation with drug-using peers only partially explains the relationship between cannabis and other illicit drug use (Fergusson & Horwood, 2000).
There is also evidence that socially deviant young people who have a predilection to use a variety of drugs including alcohol, cannabis, cocaine and heroin are selectively recruited to cannabis use (Fergusson & Horwood, 2000). The sequence of drug involvement, on this hypothesis, reflects the differing availability and societal disapproval of cannabis and other illicit drug use (Donovan & Jessor, 1983). The selective recruitment hypothesis is supported by correlations between dropping out of high school, early premarital sexual experience, delinquency, and early alcohol- and illicit drug-use. Regular cannabis users are more likely than their peers to have a history of all these behaviours (Hawkins et al., 1992; McGee & Feehan, 1993). The selective recruitment hypothesis has also been supported by a simulation study by Morral et al. (2002) which showed that this model could reproduce all the relationships between cannabis and other illicit drug use described above.
The selective recruitment hypothesis has been tested in longitudinal studies by assessing whether cannabis users are more likely to report heroin and cocaine use after statistically controlling for differences between them and nonusers (e.g. Fergusson et al., 2002b; Kandel et al., 1986; Lessem et al., 2006; Yamaguchi & Kandel, 1984). Generally, adjustment for these pre-existing differences weakens but has not eliminated the strong relationships between early and regular cannabis use and an increased risk of using other illicit drugs (see Hall & Lynskey, 2005 for a review).
Behaviour genetic studies have tested an alternative explanation of the association between cannabis and other illicit drug use: that there is a shared genetic vulnerability to develop dependence on cannabis and other drugs (Agrawal et al., 2007). Studies of identical and non-identical twins indicate that there is a partially shared genetic vulnerability to dependence on alcohol (Heath, 1995), cannabis (Agrawal & Lynskey, 2006) and tobacco (Han et al., 1999; True et al., 1999). Lynskey et al. (2003) tested this hypothesis by assessing the relationship between cannabis and other illicit drug use in 136 monozygotic and 175 dizygotic twin pairs in which one twin had, and the other twin had not, used cannabis before the age of 17 years. They found that the twin who had used cannabis was more likely to have used sedatives, hallucinogens, stimulants and opioids than their co-twin who had not. These relationships persisted after controlling for environmental factors that predicted an increased risk of developing drug abuse or dependence. A similar finding has been reported in a study of Dutch twins (Lynskey et al., 2006).
Animal studies suggest a number of ways in which the pharmacological effects of cannabis use could predispose cannabis users to use other illicit drugs (Nahas, 1990). Firstly, cannabis, cocaine, heroin and nicotine all act on the same brain “reward centre” in the nucleus accumbens (Gardner, 1999). Secondly, the cannabinoid and opioid systems in the brain interact with each other (Manzanares et al., 1999; Tanda et al., 1997). Thirdly, mutant mice in which the cannabinoid receptor had been “knocked out” do not find opioids rewarding (Ledent et al., 1999)
Animal studies also potentially provide direct tests of whether these neural mechanisms may explain the relationship between cannabis and other illicit drug use in humans. Specifically, they can assess whether administration of cannabinoids “primes” animals to self-administer other illicit drugs (Zimmer & Morgan, 1997). Two studies in rats (Cadoni et al., 2001; Lamarque et al., 2001), for example, have provided some evidence for cross-sensitivity between cannabinoids and opioids (Lamarque et al., 2001).Their relevance to adolescent cannabis use is uncertain, however, because these effects were produced by injecting large doses of cannabinoids (Lynskey, 2002).
Cannabis use is more strongly associated with other illicit drug use than alcohol or tobacco use, and the earliest and most frequent cannabis users are the most likely to use other illicit drugs. Animal studies provide some biological plausibility for a causal relationship between cannabis and other types of illicit drug use. Well-controlled longitudinal studies suggest that selective recruitment to cannabis use does not wholly explain the association between cannabis use and the use of other illicit drugs. This is supported by discordant twin studies which suggest that shared genes and environment do not wholly explain the association. Nonetheless, it has been difficult to exclude the hypothesis that the pattern of use reflects the common characteristics of those who use cannabis and other drugs (Macleod et al., 2004).
CANNABIS USE AND MENTAL HEALTH
Psychosis and schizophrenia
Cannabis use and psychotic symptoms are associated in general population surveys (see Degenhardt & Hall, 2006 for a review), and the relationship persists after adjusting for confounders (e.g. Degenhardt & Hall, 2001). The best evidence that these associations may be causal comes from longitudinal studies of large representative cohorts.
One of the earliest prospective studies of cannabis use and schizophrenia was a 15-year follow-up of 50,465 Swedish conscripts. It found that those who had tried cannabis by age 18 were 2.4 times more likely to be diagnosed with schizophrenia than those who had not (Andréasson et al., 1987). The risk increased with the frequency of cannabis use. Although substantially reduced, it remained significant after statistical adjustment for confounding variables. Those who had used cannabis 10 or more times by age 18 were 2.3 times more likely to receive a diagnosis of schizophrenia than those who had not.
Zammit et al. (2002) reported a 27-year follow-up study of the same Swedish cohort. They also found a dose-response relationship between frequency of cannabis use at age 18 and risk of schizophrenia during the follow-up. They also demonstrated that the relationship persisted after statistically controlling for the effects of other drug use and other potential confounding factors. They estimated that 13% of cases of schizophrenia could be averted if all cannabis use were prevented.
Zammit et al.’s findings have been supported by other longitudinal studies. A three-year longitudinal study of the relationship between self-reported cannabis use and psychosis in a sample of 4848 people in the Netherlands (van Os et al., 2002) found a dose-response relationship between cannabis use at baseline and psychotic symptoms during the follow-up period that persisted after statistically controlling for the effects of other drug use. Henquet et al. (2004) reported a 4 year follow-up of a cohort of 2437 adolescents and young adults between 1995 and 1999 in Munich, which found a dose-response relationship between self-reported cannabis use at baseline and the likelihood of reporting psychotic symptoms at follow up. Arseneault et al. (2002) found a relationship between cannabis use by age 15 and an increased risk of psychotic symptoms by age 26 in a prospective study of a New Zealand birth cohort. Fergusson, Horwood and Swain-Campbell (2003) reported similar findings in a longitudinal study of the Christchurch birth cohort in New Zealand. Cannabis dependence at age 18 predicted an increased risk of psychotic symptoms at age 21 years (RR of 2.3), which was reduced but still significant after adjustment for potential confounders (RR of 1.8).
Moore et al. (2007) conducted a meta-analysis of these longitudinal studies and reported an odds ratio of 1.4 [95% CI: 1.20, 1.65] of psychotic disorder among those who had ever used cannabis. There was also a dose-response relationship between frequency of cannabis use and the risk of developing psychotic symptoms or a psychotic disorder. Reverse causation was controlled in the majority of these studies by either excluding cases reporting psychotic symptoms at baseline or by statistically adjusting for pre-existing psychotic symptoms. The common causal hypothesis was harder to exclude in all studies; the association between cannabis use and psychosis was attenuated after statistical adjustment for some potential confounders, and no study assessed all major potential confounders.
Has the incidence of schizophrenia, particularly early-onset acute cases, changed over the period when there have been very substantial increases in cannabis use among young adults in Australia and North America? A study modelling trends in the incidence of psychoses in Australia did not find clear evidence of an increase in incidence following steep increases in cannabis use during the 1980s (Degenhardt et al., 2003). A similar study in Britain (Hickman et al., 2007) suggested that it may be too early to detect any effect that cannabis use has on the incidence of psychoses in the UK, because its use only increased during the 1990s. The latter study estimated that in order to prevent one case of schizophrenia in British men aged 20 to 24, we would need to prevent 5,000 men from ever smoking cannabis. The evidence from recent studies attempting to detect an increase has been mixed: one British (Boydell et al., 2006) and a Swiss study (Ajdacic-Gross et al., 2007) reported increased incidence of psychoses among males in recent birth cohorts but another British study did not find any increase (Advisory Council on the Misuse of Drugs, 2008).
A study that found an interaction between cannabis use and a common polymorphism in the COMT Val158Met allele has suggested a biological basis for the relationship between cannabis use and psychosis (Caspi et al., 2005). Alterations in catecholamine, particularly dopamine, metabolism have been documented in persons with schizophrenia (Bilder et al., 2004), and the COMT functional polymorphism is very important for the metabolism of dopamine (Mannisto & Kaakkola, 2006). This suggestive finding has not been replicated, however, in a larger case-control study of schizophrenia and cannabis use in the United Kingdom (Zammit et al., 2007).
There is also some experimental support for a direct effect of cannabis on psychotic symptoms from a provocation study by D’Souza et al. (D'Souza, 2007; D'Souza et al., 2005; D'Souza et al., 2004). In this study intravenous THC given under double-blind placebo controlled conditions produced dose-dependent increases in positive and negative psychotic symptoms in patients with schizophrenia in remission.
Cannabis use and affective disorders
Studies have found mixed relationships between cannabis use and depression. Kandel's early cross-sectional study found that cannabis use was associated with lower life satisfaction and with having consulted a mental health professional or been hospitalised for a psychiatric disorder (Kandel, 1984). Longitudinal analyses of this cohort found weaker associations between adolescent drug use and adult mental health problems (Kandel et al., 1986). Newcombe and Bentler (1988) found strong relationships between adolescent drug use and emotional distress in adolescence, but there were no relationships between adolescent drug use and emotional distress, depression and lack of a life purpose in young adulthood.
Fergusson and Horwood (1997) found a dose-response relationship between frequency of cannabis use by age 16 and a DSM-IV anxiety and depressive disorder but these relationships were no longer statistically significant after adjusting for confounding factors. Brook, Cohen and Brook (1998) reported that early cannabis use did not predict an increased risk of anxiety and affective disorders in young adulthood. McGee, Williams, Poulton and Moffit (2000) reported much the same in a longitudinal study of cannabis use and mental health in a New Zealand birth cohort. Cannabis use at age 15 did not predict mental health problems at age 18.
A number of studies have found associations between adolescent cannabis use and depression. A survey of a representative sample of Australians aged 13-17 years found that those who had used cannabis were three times more likely than those who had never used cannabis to meet criteria for depression (Rey et al., 2002). Fergusson and Horwood (1997) found that 36% of adolescents who had used cannabis 10 or more times by the age of 15-16 years met criteria for a mood disorder at that age, compared with only 11% of those who had never used cannabis. Similarly, the Zurich cohort study of young people followed from 20 to 30 years of age found that by age 30 years, those who had ever met criteria for depression were 2.3 times more likely to report weekly cannabis use (Angst, 1996). A study by Patton and colleagues (2002) of a cohort of young adults (aged 20-21 years) in Victoria found that 68% of females who reported daily cannabis use in the past year were depressed.
A meta-analysis of these studies by Moore et al. (2007) found an association between cannabis use and depressive disorders that was similar to the relationship between cannabis use and psychosis (OR = 1.49 [95% CI: 1.15, 1.94]). They argued, however, that the studies of depression and anxiety disorders had not been as well controlled for potential confounders. Nor had they convincingly excluded the possibility that young people who are depressed are more likely to use cannabis to medicate depressed feelings. They did not rule out the possibility of a relationship, because many of these studies were too small to detect any effect of cannabis use on depression and anxiety disorders.
Henquet et al. (2006) reported a three-year longitudinal study of the relationship between self-reported cannabis use and symptoms of mania in the NEMESIS community sample of 4848 people in the Netherlands. Their findings on mania substantially replicated their results on schizophrenia. Firstly, cannabis use at baseline predicted an increased risk of manic symptoms during the follow-up period in individuals who had not reported symptoms at baseline. Secondly, there was a dose-response relationship between frequency of cannabis use at baseline and risk of manic symptoms during the follow-up. Thirdly, these relationships persisted when they statistically controlled for the effects of personal characteristics and other drug use.
Suicide
A small number of studies have found a relationship between cannabis use and suicide among adolescents (see Hillman et al., 2000 for a review), but it remains unclear whether this is explained by other risk factors. In the US National Comorbidity Survey there was an association between self-reported suicide attempts and the dependence on alcohol, sedatives, stimulants, cannabis, and inhalants (Borges et al., 2000). The risk for cannabis dependence was still significant after adjusting for socio-demographic factors and other psychiatric disorders (OR of 2.4). Beautrais, Joyce and Mulder (1999) reported a case-control study of drug use in serious suicide attempts that resulted in hospitalisation. They found that 16% of the 302 suicide attempters had a cannabis use disorder (cannabis abuse or dependence), compared with 2% of the 1028 controls from the community. Controlling for social disadvantage and depression or alcohol dependence substantially reduced but did not eliminate the association (OR of 2).
The evidence from a small number of prospective studies is more mixed. Fergusson and Horwood (1997) also found a dose response relationship between frequency of cannabis use by age 16 and the likelihood of reporting a suicide attempt, but the association did not persist after controlling for confounding factors. Patton et al. (1997) found that cannabis was associated with self-harmful behaviour among females but not males, after controlling for depression and alcohol use. Andréasson and Allebeck (1990) reported in their follow up of 50,465 Swedish conscripts that the risk of suicide was four times higher among heavy cannabis users.
Moore et al.’s (2007) meta-analysis of longitudinal studies of the effects of cannabis use reported that studies of suicide were too heterogeneous to allow combination into an overall estimate of risk. The Odds Ratios (OR) in these 5 studies varied from a high of 4.6 to a low of 0.6. Few of them were able to exclude reverse causation or properly control for confounding variables, and the one study that had controlled for plausible confounders found that the relationship was no longer significant after statistical adjustment.
THE EFFECTS OF INCREASED THC IN CANNABIS PRODUCTS
Since the early 1970s concerns have been recurrently expressed that cannabis products are becoming more potent (and therefore more harmful to health) than was previously the case (Hall & Swift, 2000; McLaren et al., 2008). Regular monitoring of cannabis products in the USA indicates that THC content has increased from less than 2% in 1980 to 4.5% in 1997 (ElSohly et al., 2000) and more recently to 8.5% (ElSohly, 2008; ONDCP, 2007; see also Chapter 3). THC content also increased in the Netherlands between 2000 and 2005 (Pijlman et al., 2005), and may also have increased in other European countries - although it is uncertain by how much in the absence of time series data on THC in representative samples of cannabis products (EMCDDA, 2004). Increases in potency have probably resulted from a combination of selective breeding of higher potency plants and a shift to indoor cultivation using the sinsemilla method. All of these trends have been encouraged by the illegal status of the product, which favours the production of more concentrated forms.
The effect of any increase in the potency of cannabis products on health will depend on the extent to which users are able to offset the effects of increased THC by titrating the dose of THC that they obtain (Hall & Swift, 2000). One can conjecture about some of the possible effects of increased cannabis potency. Among naive users, higher THC content may increase the likelihood of adverse psychological effects, such as anxiety, depression and psychotic symptoms. These may discourage first-time users from continuing to use the drug. Among continued users, increased potency might increase the risk of dependence. If regular users fail to fully compensate for increased potency by titrating their dose, this would increase the risk of psychotic symptoms in vulnerable users. Any adverse effects of cannabis smoking on the respiratory and cardiovascular systems may be reduced if regular users are able to titrate to a desired dose of THC. Increased potency could also plausibly increase the risk of road traffic crashes if users do not titrate and drive while intoxicated.
The increased THC content of cannabis may not be the only relevant consideration; changes in the ratio of THC to CBD in cannabis may also be important. Potter et al. (2008) found that cannabis grown using the sinsemilla method has the highest THC: CBD ratio and cannabis resin the lowest. Given suggestive evidence that CBD has anxiolytic and anti-psychotic properties (Morgan & Curran, 2008), research needs to investigate the effect that changes in the THC: CBD ratio have had on the risk of adverse psychological effects from using cannabis.
THE ADVERSE HEALTH EFFECTS OF CANNABIS AND OTHER DRUGS
Comparisons of Cannabis with Other Drugs
How do these potential harms compare with those of other psychoactive substances in non-medical use?
One important dimension of dangerousness or harm is the likelihood of a fatal overdose (See column 1 of Table 2.1) (Gable, 2004). The “safety ratio” is the ratio between “the usual effective dose for non-medical purposes” and the usual lethal dose. Cannabis was in the lowest-risk group on this scale, along with other substances that have a ratio of 100 or higher.
Another dimension of dangerousness is the level of intoxication produced by the substance. This is influenced by the dose used, and the set and setting in which it is consumed. Nonetheless, there are differences in the propensity of different psychoactive substances to intoxicate users. The second column of Table 2.1 shows rankings made by Henningfield and Benowitz on this dimension (Hilts, 1994). Cannabis was ranked as more intoxicating than tobacco, but less so than alcohol, cocaine and heroin.
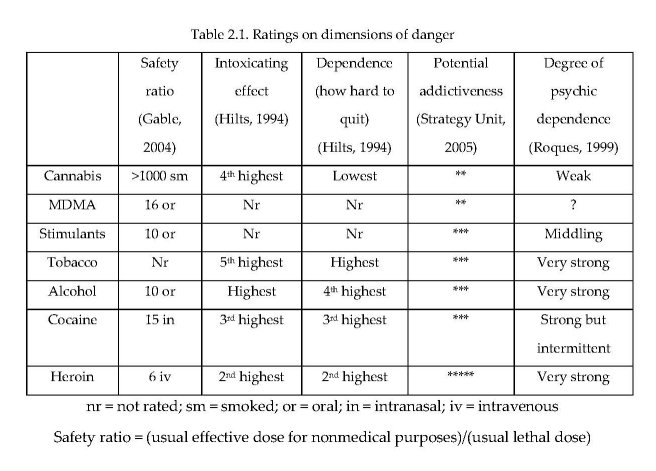
Ratings of the dependence potential or addictiveness of different substances (e.g. Hilts, 1994) compare drugs on withdrawal, tolerance, reinforcement and dependence. The report of the UK Prime Minister’s Strategy Unit (2005) rated drugs on their “potential addictiveness” and a French committee chaired by Bernard Roques (1999) rated them on “psychic dependence” (see last three columns of Table 2.1). Although there is some disagreement in the rankings for other drugs, each placed cannabis at the lowest level for the substances in the table.
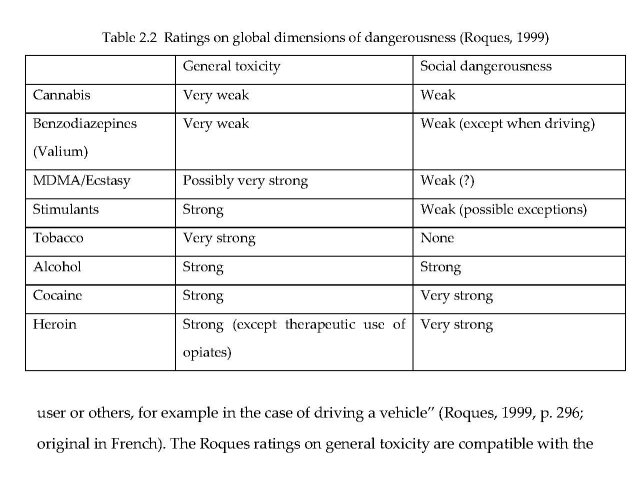
The Roques committee took a more global approach to rating dangerousness. Table 2.2 shows the committee’s rankings on “Toxicité générale” (general toxicity) and “Dangerosité sociale” (social dangerousness). In the Roques report, “toxicity” included long-term health effects such as cancer and liver disease, infections, other consequences of use, and acute effects represented by the safety ratio. The concept of social dangerousness focused on “states of comportment which can generate very aggressive and uncontrolled conduct ... induced by the product or varied disorders (fights, robberies, crimes ... ) in order to obtain it and risks for the user or others, for example in the case of driving a vehicle” (Roques, 1999, p. 296; original in French). The Roques ratings on general toxicity are compatible with the safety ratios of Gable (2004), and the social dangerousness ratings are similar to Henningfield and Benowitz’s ratings of intoxicating effect (Hilts, 1994). Cannabis is ranked “weak” on general toxicity, and “very weak” on social dangerousness.
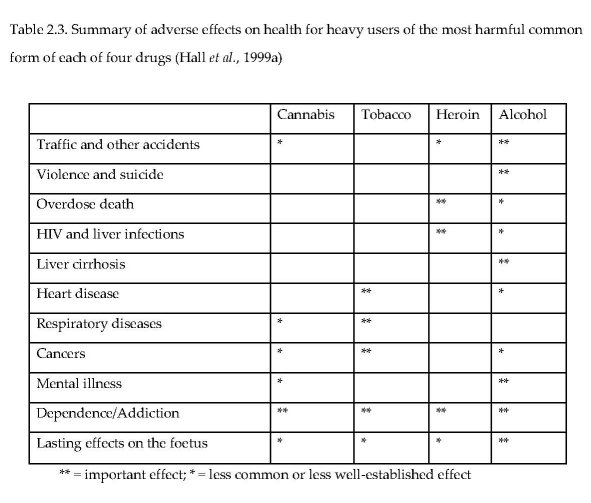
Hall et al. (1999a) compared four substances on whether there was “important effect” or a “less common or less well-established effect” on each of 11 dimensions (Table 2.3). According to these rankings, alcohol clearly has the greatest potential for harm while cannabis had the lowest number of asterisks among the four substances rated.
Nutt et al. (2007) used the ratings of experts to arrive at a global rating of the comparative harm of different drugs. They identified three main factors that determined the harms of different drugs: (i) the physical harm to the individual user; (ii) the tendency of the drug to induce dependence; and (iii) the effect of drug use on families, communities, and society. Within these categories, they recognised three components, to create a 9-category “matrix of harm”.
Physical harms were split into “acute”, “chronic”, and “intravenous” harm. Dependence was split into “intensity of pleasure”, “psychological dependence” and “physical dependence”. Social harms were split into “intoxication”, “other social harms” and “health care costs”. Expert panels of psychiatrists, pharmacologists, and addiction specialists were asked to give scores, from zero to three, for each category of harm for 20 different drugs. Cannabis was rated at eleventh most harmful out of 20 substances. Heroin and cocaine were rated the most harmful, while alcohol and tobacco, the benzodiazepines and amphetamines were rated more harmful than cannabis. Cannabis was scored well below the midpoint of scores on most dimensions. It scored above the midpoint only on intensity of pleasure, intoxication, and chronic physical harm.
The Public Health Impact Of Cannabis Use
Comparisons of the public health burden of cannabis with those of alcohol, tobacco and other illicit drugs have rarely been attempted because of dearth of evidence on impact on mortality and morbidity (Hall et al., 2008 in press; Hall et al., 2006). One of the earliest attempts (Hall, 1995) made a qualitative assessment that identified the most important public health impacts of cannabis use “in order of approximate public health importance” as: motor vehicle accidents; cannabis dependence; respiratory disease; precipitation and exacerbation of schizophrenia in vulnerable individuals; low birth weight babies; and subtle cognitive impairment.
The most recent estimate of the contribution of illicit drugs to the global burden of disease (BOD) confined itself to estimating the contribution of illicit opioid use, because these drugs had the best epidemiological evidence of adverse impact on mortality. Studies estimating the economic costs of alcohol, tobacco and illicit drugs have often not disaggregated the effects of cannabis from those of opioids (e.g. Collins & Lapsley, 2007). One recent study that did disaggregate cannabis (Rehm et al., 2007) only counted morbidity that could be directly attributed to cannabis via a diagnostic code, namely, episodes of hospital care for cannabis dependence.
A recent Australian study did make a more serious attempt to estimate the contribution that cannabis use made to the burden of disease (BOD) in Australia (Begg et al., 2007). This study included estimates of disability due to cannabis dependence and cannabis-related psychoses, and it also attributed small proportions of MVA deaths and suicides to cannabis use. It estimated that cannabis was responsible for 0.2% of total disease burden. This comprised 10% of the BOD attributable to the use of all illicit drugs (2.0%), a similar proportion of that due to alcohol (2.3%) but a small fraction of that due to tobacco use (7.8%). Even allowing for under-estimation, the contribution of cannabis to BOD on current patterns of use was very modest in a country with one of the highest prevalences worldwide of cannabis use in the late 1990s (UNODC, 2006). Even so, the contribution of cannabis might well rise somewhat if it was as freely available, as heavily promoted and as widely used as alcohol and tobacco are now.
SUMMARY
The acute adverse effects of cannabis use include anxiety and panic, especially in naive users, and an increased risk of accident if a person drives a motor vehicle while intoxicated with cannabis. Women who smoke during pregnancy increase their risk of giving birth to a low-birth weight baby.
The most probable adverse health effects of chronic cannabis use are increased risks of: a cannabis dependence syndrome; chronic bronchitis and impaired respiratory function in regular smokers; increased risk of cardiovascular disease in older adults who continue to smoke into middle age; respiratory cancers in very long-term daily smokers; and psychotic symptoms and disorders in heavy users - especially those with a pre-existing history of such symptoms, a family history of such disorders, or who begin use in their early teens. Among the most probable adverse psychosocial effects among adolescents who initiate early are an increased risk of cannabis dependence and impaired educational attainment. Regular adolescent cannabis users have a higher risk of using other illicit drugs, although the explanation of this relationship and its implications remain contentious.
The public health impact of contemporary patterns of cannabis use are modest by comparison with those of other illicit drugs (such as the opioids) or with alcohol. In the former case this reflects the absence of fatal overdose risk from cannabis. In the latter case, it reflects the much lower risks of death from cannabis- than alcohol-impaired driving, fewer adverse effects on health, lower rates of regular use to intoxication for cannabis than for alcohol, and the lower rate of persistence of cannabis use into older adulthood.
| < Prev | Next > |
|---|












