MDMA-Induced Neurotoxicity
Drug Abuse
MDMA-Induced Neurotoxicity
Parameters of Degeneration and
Recovery of Brain, Serotonin Neurons
GEORGE BATTAGLIA, S. Y. YEH AND ERROL B. DE SOUZA'
Neuroscience Branch, Addiction Research Center
National Institute on Drug Abuse, Baltimore, MD 21224
BATTAGLIA, G., S. Y. YEH AND E. B. DE SOUZA. MDMA-induced neurotoxicity : Parameters of degeneration and recovery of brain serotonin neurons. PHARMACOL BIOCHEM BEHAV 29(2) 269-274, 1988-This study investigates a number of parameters that influence the neurotoxic effects of 3.4-methylenedioxymethamphetamine (MDMA) on serotonin (5-HT) neurons in brain. Both the dose and number of injections of MDMA affect the degree of neurotoxicity on 5-HT axons and terminals as assessed by decreases in the content of 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) and the density of 5-HT uptake sites. Repeated systemic administration of various doses of MDMA (5-20 mg/kg twice daily for 4 consecutive days) results in dose-dependent decreases in 5-HT, 5-HIAA and 5-HT uptake sites. Increasing the number of injections of MDMA resulted in progressively greater reductions in 5-HT and 5-HIAA which occurred prior to decreases in 5-HT uptake sites. In contrast, no significant changes were observed in the density of norepinephrine uptake sites following single or repeated injections of 20 mg/kg MDMA. With respect to neuronal regeneration, following an initial 90,70 loss of 5-HT uptake sites after treatment with MDMA, the recovery of these sites occurred over a protracted period of time; a marked 25% reduction was seen at 6 months and the concentration of 5-HT uptake sites returned to control levels at 12 months following treatment with MDMA. Pretreatment with the selective 5-HT uptake blocker, citalopram, prior to each injection of MDMA prevented the neurotoxic effocts of MDMA on the 5-HT parameters described above suggesting that active uptake of MDMA or a MDMA-related subAance into brain 5-HT neurons was involved in the neurotoxic actions of the drug. In addition, the neurodegenerative effects of MDMA on 5-HT neurons exhibited some species specificity as comparable decreases in cerebral cortical 5-HT, 5-HIAA and 5-HT uptake sites were observed in rat and guinea pig while no significant changes in any of these serotonergic parameters were seen in mouse brain.
Neurotoxicity 5-HT uptake sites Regeneration 5-HT 5-HIAA
3, 4-METHYLENEDIOXYMETHAMPHETAMINE (MDMA) has recently attracted a great deal of attention due to its increasing abuse among certain segments of the population [1,11]. Recent data demonstrating that M13MA is selfadministered by both rhesus monkeys [41 and baboons [181 suggest that MDMA may have high abuse potential in man. These reports are particularly disturbing as we and others have recently demonstrated that MDMA is a potent neurotoxin that appears to cause selective degeneration of brain serotonin neurons [2, 3, 7, 22, 23, 27, 28, 30-321 comparable to that reported for 3,4-methylenedioxyamphetamine (NIDA) [2, 24, 30, 311. Typically, neurotoxic effects of drugs on 5-HT neurons have been assessed from reductions in brain levels of serotonin (5-HT) and 5-hydroxyindoleacetic acid (5-HIAA), and decreases in the maximal activity of tryptophan hydroxylase (TPH) and the serotonin active uptake carrier. Since MDMA can inhibit the activity of TPH, the ratelimiting enzyme in 5-HT synthesis [30,31], it is unclear whether the MDMA-induced reductions in 5-HT and 5-HIAA may be due to suppressed neurotransmission in otherwise structurally intact 5-HT neurons or may represent the consequence of the destruction of 5-HT axons and terminals.
Since 5-HT uptake sites are highly concentrated on 5-HT containing nerve terminals [ 17], we have taken the approach of using specific radioligands such as IHparoxetine to directly label 5-HT uptake sites in brain and to assess the neurodegeneration of 5-HT neurons by measuring changes in the density of 5-HT uptake sites. We have recently reported the feasibility of using the measurement of 3H-paroxetinelabeled 5-HT uptake sites to quantify the neurotoxic effects of MDMA on 5-HT neurons in various regions of rat brain [2.32). Visualization of MDMA-induced destruction of 5-HT axons and terminals using antibodies directed against serotonin [22.231 and preliminary autoradiographic studies demonstrating corresponding decreases in ~H-paroxetinelabeled 5-HT uptake sites in slide-mounted brain sections of MDMA-treated rats [31 further validate the use of changes in the density of 5-HT uptake sites as an appropriate index of 5-HT neurodegeneration.
The present study was designed to elucidate some of the parameters that may contribute to the marked neurotoxic effects of MDMA on brain serotonin neurons. By measuring changes in 5-HT uptake sites as well as---traditional-markers used to assess such as 5-HT and 5-HIAA content, we have assessed the neurodegenerative effects of MDMA on serotonin neurons with respect to: (1) the effects of dose and dose regimen (i.e., number of injections), (2) the time course of neuronal recovery, (3) the role of the 5-HT transporter complex and (4) the effects in various species.
METHOD
Materials
'H-Paroxetine (specific activity: 22.3 Ci/mmol), 'HMazindol (15 Ci/mmol), and Formula 963 scintillation fluid were obtained from New England Nuclear (Boston, MA). MDMA was provided by the National Institute on Drug Abuse. Mazindol and citalopram were generously provided by Sandoz Pharmaceuticals (East Hanover, NJ) and Lundbeck (Denmark), respectively. All other compounds were obtained from Sigma Chemical Co. (St. Louis, MO).
Animal Treatments
In studies investigating the dose-dependent neurotoxic effects of MDMA, groups of male Sprague-Dawley rats (Harlan Industries, IN) weighing 175-200 g were injected subcutaneously (SC) with either saline (I ml/kg) or various doses of MDMA (5-20 mg/kg expressed as the free base) twice a day (approximately every 12 hours) for 4 consecutive days. One group of rats was injected with the 5-HT uptake blocker, citalopram (10 mg/kg IP), 30 minutes prior to each SC administration of 10 mg/kg MDMA. In order to invest1gate the neurotoxic effects of single versus multiple injections of MDMA, groups of rats were injected every 12 hours with either saline (I mllkg) or MDMA (10 mg/kg SC) one, two, four, or eight times. In both studies, the animals were sacrificed 18 hours after their last injection. In studies investigating the time course of neuronal recovery from the effects of MDMA, rats were administered either saline (I ml/kg) or MDMA (20 mg/kg SC) twice a day for 4 consecutive days; groups of saline-treated and drug-treated rats were sacrificed at various times (18 hours, 1. 2, 4, 8. 26 and 52 weeks) following treatment. In studies investigating the long-term neurotoxic effects of MDMA in different species, groups of male Duncan Hartley guinea pigs (350-400 g), ICR mice (30-40 g) and Sprague-Dawley rats (175-200 g) were administered MDMA (20 mg/kg SC) twice a day for 4 consecutive days and the animals were sacrificed 7 days after the last injection. In all studies, the frontal cortex was dissected, frozen in liquid nitrogen and stored at -70'C until assayed. The frontal cortex was chosen since this brain region contains an abundance of serotonergic markers including high concentrations of 5-HT, 5-HIAA and 5-HT uptake sites [2,121.
Monoamine Determinations
Portions of frontal cerebral cortices (10-20 mg) were weighed and homogenized in I ml of 0. 1 N HCIO, containing 0.1% cysteine. After centrifugation. catecholamines, serotonin and their metabolites in the supernatant were measured with electrochemical detection after separation by reversed phase HPLC. Samples (20 /A were chromatographed on a C,, radial-pak cartridge and eluted with a mobile phase at a flow rate of 0.9 ml per minute. Each liter of the mobile phase contained 0.1 g EDTA, 1.3 g of sodium heptayl sulfonate, 8 ml of triethylamine, and 45 ml of acetonitrile and the pH was adjusted to 2.85 with phosphoric acid (-4.5 ml). Concentrations of catecholamines, 5-HT and their metabolites were calculated from standard curves prepared from at least four standards. The standard curves were linear from 3 to 100 ng/ml of monoamine or morroamine metabolites (60 to 2000 pg/20 lul). The retention times for norepinephrine (NE), dihydroxybenzylamine (DHBA) (Internal Standard), dopamine (DA), 5-HIAA, and 5-HT were 3.15, 4.5, 6.46, 13.52, and 17.45 min, respectively.

FIG. 1. The effect of repeated systemic administration of various doses of MDMA on the content of serotonin (5-HT) and 5-hydroxyindoleacetic (5-HIAA) and on the density of 5-HT uptake sites in frontal cortex. Rats were administered saline or MDMA I" ice a day for 4 consecutive days and sacrificed 18 hours after the last injection. One group of rats was pretreated with 10 mg/kg citalopram 30 minutes prior to each administration of 10 mg/kg MDMA. Data represent the meantS.E.M. from 3-5 rats and are expressed as a percent of values in control, saline-injected rats. Control values for 5-HT and 5-HIAA levels were 387:L61 and 15 1 -20 pg/mg tissue, respectively. The density of 5-HT uptake sites in the frontal cortex in controls was 396- 15 fmol/mg protein. Data were analyzed by oneway ANOVA and Duncan's multiple range test. *, ** and *** indicate significant differences atp<0.05,p<0.0I and p<0.001, respectively, from control saline-treated rats. 't and i ', t indicate significant differences at p<0.01 and p<0.001, respectively. from all other groups.
Measurement of Monoamine Uptake Sites
Frontal cortex from individual saline- and drug-treated animals was weighed and prepared as previously described [2,12]. Tissues were placed in 50 volumes of ice-cold 50 mM Tris-HCI, 120 mM NaCl, 5 mM KCI (pH 7.4 at 22'C) and homogenized using a Brinkman polytron (setting of 5, 2x30 sec). The homogenate was centrifuged at 48,000xg for 10 min with a subsequent resuspension and wash of the pellet. The final pellet was resuspended in the same buffer to a concentration of 15 mg wet weight/mi. Previous studies [2] indicated that there was no significant difference in the affinity (i.e., K,) of "H-paroxetine-labeled 5HT uptake sites between control and MDMA-treated rats. To obtain an estimate of the maximal density of IH-paroxetine-labe led 5-HT uptake sites a saturating concentration (0.25 nM) of 1Hparoxetine (Kn=0.01 nM) was incubated with 1.~ mg of tissue in the absence and presence of I tiM citalopyam in 5 ml of the assay buffer. The contents were incubated for 2 hr at 22'C and then rapidly filtered over polyethyleneimine (PEI)-presoaked Whatman GF/C filters and washed with 15 ml of buffer. Filters were then equilibrated,with 5 ml of Formula 963 scintillation fluid and counted at 54% efficiency in a Beckman 3801 scintillation spectrometer. Proteins w ere determined according to the method of Lowry et al. [19].
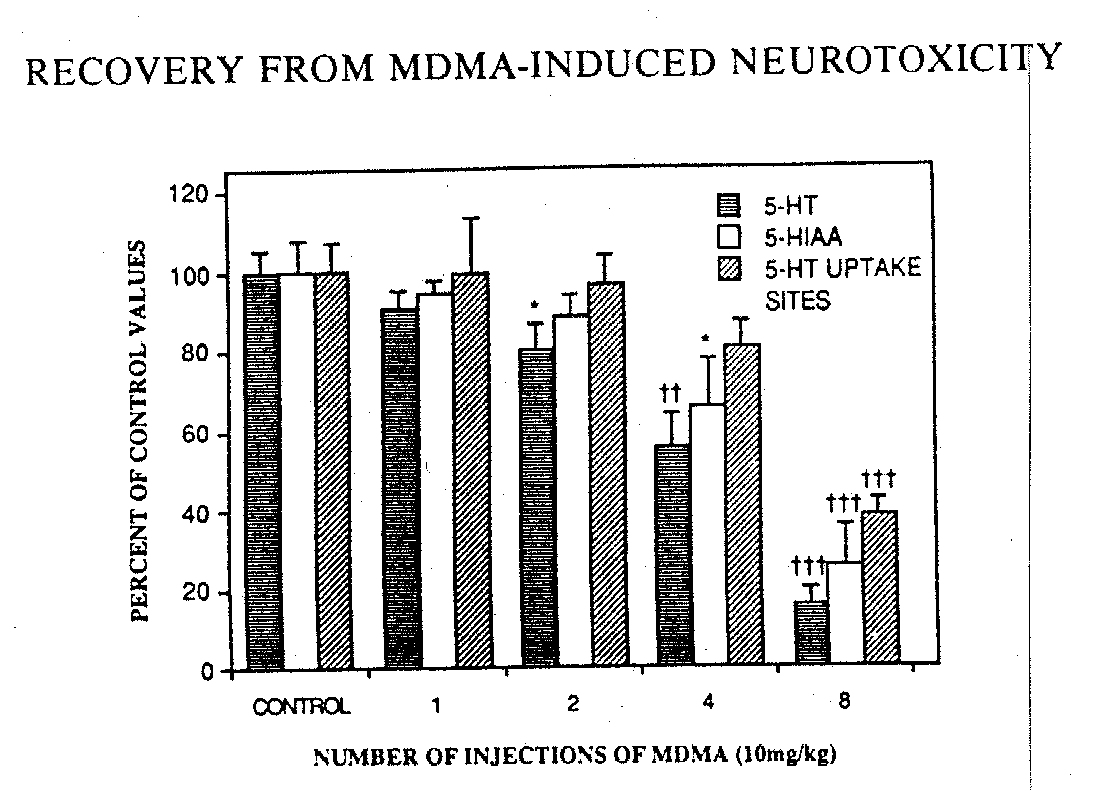
FIG. 2. The effects of single and multiple injections of MDIXIA on the content of serotonin (5-HT) and 5-hydroxyindoleacetic acid (5HIAA) and on the density of 5-HT uptake sites in rat frontal cortex. Rats were injected the specified number of times with either saline or 10 mg/kg MDMA and sacrificed 18 hours after the last injection. Data which represent the mean and S.E.M from three to five animals are plotted as a percent of respective values for each of the parameters in control, saline-injected rats. Control levels of 5-HT and 5-HIAA were 475±24and 332--t24 pmol/mg tissue. respectiNely. The density of 5-HT uptake sites was 349±24 fmol/mg protein in controls. Data were analyzed by one-way ANOVA and Duncan's multiple range test. * indicates a significant difference at p<0.05 from corresponding control saline-injected rats: -I" and - 1 -1" indicate significant differences at p<0.01 andp<0.001, respectively, from all other groups.
NE uptake sites were measured using 6 nM IH-mazindol and 0.3 uM desipramine to define specific binding as previously described [141. Assays were carried out in 0.5 ml of 50 mM Tris-HCI, 120 ImM NaCl, 5 mM KCI (pH 7.4 at 22'C). Tubes containing drugs and tissue (5-8 mg wet weight/tube) were incubated on ice for 60 min then the contents were rapidly filtered over Whatman GF/C filters which had been presoaked in 0.059c PEI and washed with 15 ml of cold buffer.
RESULTS
Dose Dependence
As shown in Fig. 1, a repetitive dosing regimen (i.e., twice a day for 4 consecutive days) of MDMA at various doses up to 20 mg/kg resulted in dose-dependent decreases in the content of 5-HT and 5-HIAA and in the density of 5-HT uptake sites in rat frontal cortex at 18 hours following the last injection. At the lowest dose of MDMA tested (5 mg/kg). 5-HT content was markedly reduced (45%), while only a small (14cl'c), but statistically significant, decrease in the density of 5-HT uptake sites was observed: a small decrease in 5-HIAA content was also observed at this dose. although this change was not statistically significant. Higher doses of MDMA (10 and 20 mg/kg) resulted in comparable reductionsin 5-HIAA levels (60~7Wc), while the decrease in 5-HT was significantly greater at 20 mg/kg (9Wc) than at 10 mg/kg (8Wc). The density of 5-HT uptake sites decreased progressively as the dose of MDMA was increased with a maximal reduction of 90% observed at 18 hours following administration of 20 mg/kg MDMA. In contrast, following a treatment regimen of 20 mg/kg MDMA, there were no significant differences in the density of :'H-mazindol-labeled NE uptake sites (fmol/mg protein) in the frontal cortex between saline( 159±17) and drug- (152±5) treated animals.
FIG. 3. Time course of recovery of serotonin (5-HT) uptake sites in rat frontal cortex following repeated systemic administration of XIDMA. Rats were treated with either saline or 20 mg/kg MDMA twice a day for 4 consecutive days and then sacrificed at various times following the last injection up to 12 months. Salineinjected control rats were killed at each of the time points, and the data Ahich represent the mcan±S.E.M. of five rats per group are plotted as a percent of the value of control animals at the respective time.
single versus Mulliple Injections
Since repeated systemic administration of 10 mg/kg NIDMA caused marked neurodegeneration of cortical 5-HT neurons , we chose to investigate the neurodegenerative effects of single versus multiple injections of MDMA at this dose. As shown in Fig. 2. increasing the number of injections of MDMA (10 mg/kg SC) resulted in significant and progressively greater reductions in 5-HT and 5-HIAA content. While one injection of MDMA was without effect on any of the serotonergic parameters examined, two doses were sufficient to elicit a significant reduction (-210c) in 5-HT content. A significant reduction (-34%) in 5-HIAA content was observed only after four injections of MDMA. Marked reductions of 84% and 75%, in 5-HT and 5-HIAA, respectively, were observed following an eight injection regimen of 10 mg/kg MDMA: the density of 'Hparoxetine-labeled 5-HT uptake sites was only significantly decreased (-64%) following eight injections of MDMA at this dose. While there was no change in the DA content in any of the groups, a small and consistent decrease in NE content (-2Wc) was observed in all MDMA-treated rats. This small change in NE following MDMA treatment was not accompanied by a reduction in the density of :'H-mazindol-labe led NE uptake sites (data not shown).
Time Course of Regeneration of 5-HT Uptake Sites
Although we and others have previously reported that the neurodegenerative effects of MIJIMA on 5-HT neurons can be observed up to 14 days after treatment, the time course of recovery of affected 5-HT neurons remains to be demonstrated. As shown in Fig. 3, we have investigated the time course of neuronal regeneration by measuring the recovery of 5-HT uptake sites in rat frontal cortex up to 12 months following repeated systemic administration (i.e., twice daily SC injections for 4 days) of 20 mg/kg MDMA. At all time points up to 6 months during the recovery time course, the density of 5-HT uptake sites was significantly below the corresponding values in age-matched saline-treated controls. At the 6 month time point, the density of 5-HT uptake sites was only 75% of the values of saline-treated controls whereas by 12 months after MDMA treatment the density of 5-HT uptake sites returned to control levels. The shape of the recovery curve suggests that there may be a faster initial rate of recovery of 5-HT uptake sites that occurs between 18 hours and 4 weeks which is followed by a slower rate of recovery which occurs between 4 weeks and 12 months. These data indicate that more than 6 months are required for a complete recovery of 5-HT uptake sites to control levels.
Effect of 5-HT Uptake Site Blockade
As the neurotoxic effects of drugs such as parachloroamphetamine on 5-HT neurons can be prevented by 5-HT uptake blockers [25,26], we investigated if the 5-HT uptake carrier protein was likewise involved in the neurotoxic effects of MDMA. As shown in Fig. 1, pretreatment of rats with the selective 5-HT uptake blocker, citalopram (10 mg/kg), prior to each injection of 10 mg/kg MDMA, resulted in nearly complete protection against the neurotoxir.* effects of MDMA. Citaloprampretreated rats exhibited only a 15% decrease in 5-HT uptake sites following MDMA treatment in comparison with a 6(Fc reduction in 5-HT uptake sites observed in rats treated with an identical dose of MDMA alone. In addition, citaloprarn pretreatment completely prevented the MDMA-induced decreases in the concentration of both 5-HT and 5-HIAA.
Species Specificity
Since amphetamine has been shown to be metabolized by different pathways in rat, mouse and guinea pigs [6], additional studies were carried out to investigate whether MIJIMA was also neurotoxic in mouse and guinea pig. Animals in these studies were treated repeatedly (i.e., twice a day for 4 consecutive days) with 20 mglkg MDMA and levels of 5-HT, 5-FI1AA and 5-HT uptake sites were measured 7 days later to assess the long-term effects of the treatment. As shown in Fig. 4, MDMA caused comparable and marked decreases in 5-HT and 5-HIAA content and in the density of 5-HT uptake sites in rat and guinea pig cortex, but was without effect on any of these serotonergic parameters in the mouse.
DISCUSSION
Previous studies have demonstrated that the repeated systemic administration of MDMA is neurotoxic to 5-HT neurons in rat brain [2, 3, 7, 22, 23, 27, 28, 30-321. The present study was designed to investigate the influence of certain treatment parameters on the neurotoxic effects of MIJIMA and to determine if these changes are reversible. We report that the neurotoxic actions of MDMA on serotonergic neurons appear to be a function of both the dose administered as well as the number of times the drug is given. Furthermore, the dose and/or number of injections of MDMA can differentially affect the various serotonergic markers used to assess neu rode generation. With respect to the dose of MIJIMA, 5-HT levels appear to be more readily decreased (45% reduction at 5 mg/kg) while 5-HIAA levels are only significantly decreased (-60,7c) following four or eight doses of either 10 or 20 mglkg MDMA. We have previously demonstrated that in either homogenates [2,321 or slidemounted brain sections [31, the loss of 5-HT uptake sites provides an appropriate index of the degeneration of serotonergic neurons in that this measure corresponds to the degree of loss of neuronal axons and terminals detected using 5-HT immunocytochemical techniques [22,231. By using decreases in the density of 5-HT uptake sites as an index of neurodegeneration, it is apparent that MDMA causes the dose-dependent destruction of cortical 5-HT axons and terminals with a significant decrease observed even at the lowest dose of MDMA employed (5 mg/kg). These results demonstrate the dose-dependence of 5-HT neurotoxicity following a repetitive injection regimen of MDMA.
FIG. 4. The effects of repeated systemic administration of MDMA on the content of serotonin (5-HT) (A), 5-hydroxyindoleacetic acid (5-HIAA) (13) and the density of 5-14T uptake sites (C) in rat, guinea pig and mouse frontal cortex. Animals were treated with saline or 20 mg/kg MDMA twice a day for 4 consecutive days then sacrificed 7 days after the last injection. Data represent the meant S.E.M. of five animals per group and are expressed as a percent of safine-injected control values in the respective species. In rat, guinea pig and mouse, control values of 5-HT were 275:L41, 296tI4 and 449t36 pg/mg tissue, respectively; control values of 5-HIAA were 345t4O, 92t4 and 319t34 pg/mg tissue, respectively, control values of 5-HT uptake sites were 397tI0, 216t6 and 233tI2 fmoUmg protein, respectively. Data were analyzed by Students t-test. *** indicates a significant difference at p <0.001 from respective control values.
Since MDMA has been reported to increase the release of serotonin [15,21] and cause suppression of tryptophan hydroxylase activity following systemic administration [30,311, it is important to differentiate between the suppressive effects of MDMA on 5-HT metabolic processes in structurally intact neurons and the neurodegenerative effects of MDMA on the structural integrity of 5-HT axons and terminals. We have addressed this question by comparing the effects of single versus multiple injections of MDMA on each of the serotonergic parameters described. The correspondence between changes in 5-HT or 5-HIAA content and decreases in the density of 5-HT uptake sites, which is indicative of 5-HT neurodegeneration, can be used to assess the relationship between changes in metabolic activity and actual destruction of neurons following treatment With M13MA. These studies (Fig. 2) demonstrating changes in 5-HT and 5-HIAA levels following two and four injections of MDMA but no significant decrease in 5-HT uptake sites until eight repetitive injections of the drug suggest that M13MAinduced changes in 5-HT metabolic activity can precede and may occur independent of the processes determining destruction of 5-HT axons and terminals.
To date, no investigations have reported whether 5-HT neurons can recover from the neurotoxic effects of MDMA and what the time course of neuronal recovery might be. We report here a recovery time course covering a 6-month period following administration of MDMA which indicates that cortical 5-HT axons and terminals can undergo a regeneration process subsequent to an initial 9Wo reduction. The time course of recovery in the frontal cortex, however, occurs over an extremely long period of time as there is a 25% deficit in 5-HT uptake sites up to 6 months and a return to control values at 12 months after MDMA treatment. The persistent neurotoxic effects on 5-HT neurons following MDMA is similar to that observed following parachloroamphetamine (PCA) in which marked reductions in 5-HT have been observed up to 4 months after a single injection [8, 9, 16, 261. As we have used the recovery of 5-HT uptake sites as an index of neuronal regeneration, it is unclear from the present data whether there is an actual regeneration of neurons that have previously undergone axon or terminal degeneration or whether the increased density of uptake sites is a consequence of increased collateral sprouting of neurons unaffected by the drug treatment. It has previously been reported that following 5,6-dihydroxytryptamineinduced axotomy, axonal sprouting occurs within 4-5 days and the appearance of new axonal sprouts correlates with the recovery of 3 H-5-HT uptake [51. Evidence from both immunocytochemical data [22,231 and autoradiographic studies of 5-HT uptake sites [31 indicating that 5-HT cell bodies appear to be insensitive to the neurotoxic effects of repeated systemic administration of MDMA provide a mechanism by which terminal regeneration of MDMA-affected neurons may occur.
The data of the present study demonstrate that destruction of 5-HT axons by MDMA involves the 5-HT active uptake carrier as blockade of this carrier by administration of citalopram, a selective 5-HT uptake blocker [131, prior to administration of MDMA can prevent the decreases in 5-HT markers elicited by MDMA alone (see Fig. 1). These data are consistent with previous reports for other potent 5-HT neurotoxins demonstrating that pretreatment with 5-HT uptake blockers can prevent the neurotoxic effects of PCA [25,26]. It has recently been shown that MDMA-induced neurotoxicity can be prevented or reversed if a 5-HT uptake blocker is administered no later than 12 hours after MDMA treatment [28].
High doses of MDMA (20 mg/kg) which markedly decrease all parameters of cortical 5-HT neurons in the rat and guinea pig produce no significant changes in the content of 5-HT and 5-HIAA and in the density of 5-HT uptake sites in the mouse. While differences in sensitivity to the neurotoxic effects of PCA on 5-HT neurons have been observed in mouse when compared to its effects in rat and guinea pig [8, 9, 16, 26], the differential sensitivity may be due, in part, to speciesdependent differences in the half-life of PCA [29]. Active neurotoxic metabolites or metabolic intermediates of PCA have previously been postulated as being responsible for its neurotoxic effects on 5-HT neurons [10,26], although to date no such active species has been identified. While there has been no direct demonstration of a neurotoxic metabolite of MDMA, some preliminary data suggest that an active metabolite of MDMA may be responsible for elicitng its neurotoxic effects. We have previously reported that in contrast to the marked 5-HT neurodegenerative effects following systemic administration of MDMA or MDA, direct intracerebral injections of MDMA or MDA are without effect on cortical 5-HT neurons as visualized directly using an antibody directed against serotonin [20]. Our observation of marked species differences in sensitivity to MDMA-induced 5-HT neurotoxicity would be consistent with the hypothesis of a peripherally produced neurotoxic metabolite of MDMA. While these findings suggest that a peripheral metabolite of MDMA may be the active neurotoxic species, in preliminary studies, we have found that in rats pretreated with the cytochrome P-450 enzyme inhibitor, SKF 525A, there was no potentiation or attenuation of the neu rodege ne ration found following repeated administration of 10 mg/kg of MDMA (unpublished observation). Additional studies are required to identify the neurotoxic species of MDMA. In summary, our findings indicate that in rat, MDMA-induced neurotoxicity of brain 5-HT terminals is dependent on the dose of drug used as well as the number of times the drug is administered. Following extensive destruction of 5-HT neurons, regeneration can occur but requires a substantial amount of time. The ability of 5-HT uptake blockers to prevent the neurotoxic effects of MDMA suggests that the active uptake process is involved in the mechanism of neurotoxicity of MDMA.
ACKNOWLEDGEMENTS
We thank Terrie Pierce and Mary Flutka for manuscript preparation. This work was supported in part from funds provided by the U.S. Food and Drug Administration.
REFERENCES
1. Adler, J., B. Abramson, S. Katz and M. Hager. Getting high on *'Ecstacy.'* Newsweek, April 15, 1985. p. 96.
2. Battaglia. G., S. Y. Yeh, E. O'Hearn, M. E. Molliver, M. J. Kuhar and E. B. De Souza. 3.4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: Quantification of neurodegeneration by measurement of 'li-paroxetine-labeled serotonin uptake sites. J Pharmacol Exp Ther 242: 911-916, 1987.
3..Battaglia, G., M. J. Kuhar and E. B. De Souza. MDMA-induced neurotoxicity visualized by in vitro autoradiography: Differential sensitivity of serotonergic neurons. Fed Proc 46: 404. 1987.
4. Beardsley, P. M., R. L. Balster and L. S. Harris. Selfadministration of methylenedioxymethamphelamine (MD.IIA) by rhesus monkeys. Drug Alcohol Depend IS: 149157, 1986.
5. Bjorklund, A., A. Nobin and U. Stenevi. Regeneration of central serotonin neurons after axonal degeneration induced by 5.6dihydroxytryptamine. Brain Res 50: 214-220, 1973.
6. Caldwell, J. The metabolism of amphetamines and related stimulants in animals and man. In: Amphetamines and Related Stimulants: Chemical, Biological, Clinical, and Sociologi((.I.Aspects, edited by J. Caldwell. Boca Raton, FL: CRC Press. 1980, pp. 29-46.
7. Commins, D. L., G. Mosmer, R. M. Virus, W. L. Woolverton, C. R. Schuster and L. S. Seiden. Biochemical and histological evidence that methylenedioxymethamphetamine (MDMA) is toxic to neurons in the rat brain. J Pharinmol Exp Ther 241: 338-345, 1987.
8. Fuller, R. W. and K. W. Perry. Long-lasting depiction of brain serotonin by 4-chloroamphetamine in guinea pigs. Brain R(s 82: 383-385, 1974.
9. Fuller, R. W. Neurochemical effects of serotonin neurotoxins: An introduction.Ann NY Acad S(i 305: 178-181, 1978.
10. Gal, E. M. and A. D. Sher-man. Cerebral metabolism of some serotonin depletors. Ann NY Acad S(i 305: 119-127, 1978
11. Gertz, K. R, The agony of ecstasy. Science D~qest, February, 1986, p. 27.
12. Habert, E., D. Graham, L. Tahraoui, Y. Claustre and S. Z. Langer. Characterization of 3H-paroxetine binding to rat cortical membranes. Ear J Pharmacol 118: 107-114. 1985.
13. Hytell, J. Citalopram; basic and clinical studies. 11rog Neuropharmacol Biol Ps ' vchialry 6: 275-336, 1982,
14. Javitch, J. A., R. 0. Blaustein and S. H. Snyder. ["H]Mazindol binding associated with neuronal dopamine and norepinephrine uptake sites. Vol Pharmacol 26: 35-44, 1984.
15. Johnson, M. P., A. J. Hoffman and D. E. Nichols,Vffects of the enantiomers of MDA, MDMA and related analogues on :'Hserotonin and IH-dopamine release from superfused rat brain slices. Eur J Pharmacol 132: 269-276, 1986.
16. Kohler, C., S. B. Ross, B. Szrebo and S. 0. Ogren. Long-term behavioral and biochemical effects of p-chloroamphetamine in the rat. Ann NY Ac ad Sci 305: 645-663, 1978.
17. Kuhar, M. J. and G. K. Aghajanian. Selective accumulation of IH-serotonin by nerve terminals of raphe neurons: an autoradiographic study. Nature 241: 187-189, 1973,
18. Lamb, R. J. and R. R. Griffiths. Self-administration of d.1 methylenedioxymethamphetarnine (MDMA) in the baboon. Ps - vchopharmacology (Berlin) 91: 268-272, 1987.
19. Lowry, 0. H., N. J. Rosebrough, A. L. Farr and R. J. Randall. Protein measurement with the folin phenol reagent. J Biol Chem 193: 265-275, 1951.
20. Molliver, M. E., E. O'Hearn, G. Battaglia and E. B. De Souza. Direct intracerebral administration of MDA and MDMA does not produce serotonin neurotoxicity. Soc Neurosci Absir 12: 1234, 1986.
21. Nichols, D. E., D. H. Lloyd, A. J. Hoffman, M. B. Nichols and G. K. Yim. Effect of certain hallucinogenic amphetamine analogues on the release of IH-serotonin from rat brain synaptosomes. J Mcd Chem 25: 530-535, 1982.
22.O'Hearn, E., G. Battaglia, E. B. De Souza, M. J. Kuhar and M. E. Molliver. Met hyle ned ioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause ablation of serotonin axon terminals in the forebrain: Immunocytochemical evidence. J Neurosci, in press, 1988.
23. O'Hearn, E., G. Battaglia, E. B. De Souza, M. J. Kuhar and M. E. Molliver. Systemic MDA and MDMA, psychotropic substituted amphetamines, produce serotonin neurotoxicity. Soc Neurosci Abstr 12: 1233, 1986.
24. Ricaurte, G., G. Bryan, L. Strauss, L. Seiden and C. Shuster. Hallucinogenic amphetamine selectively destroys brain serotonin nerve terminals. S(ien(e 229: 986-988, 1985.
25. Ross, S. B., S. 0. Ogren and L. Renyi. Antagonism of the acute and long-term biochemical effects of 4-chloramphetamine on the 5-HT neurons in rat brain by inhibitors of the 5-hydroxytryptamine uptake. A(la Pharmac ol Toxi(ol 39: 456-476, 1976.
26. Sanders-Bush, E. and L. R. Steranka. Immediate and long-term effects ofp-chloroamphetamine on brain amines. Ann NYAcad S(i 305: 208-221, 1978.
27. Schmidt, C. J., L. Wu and W. Lovenberg. Methylenedioxymethamphetamine: A potentially neurotoxic amphetamine analogue. Eur J Pharmacol 124: 175-178, 1986.
28. Schmidt. C. 1. Neurotoxicity of the psychedelic amphetamine. met hyle nediox ymethamphetami ne. J Pharmacol Exp Ther 240: 1-7, 1986.
29. Steranka, L. R. and E. Sanders-Bush. Long-term effects of continuous exposure to p-chloramphetamine on central serotonergic mechanisms in mice. Bio(hein Pharmacol 27: 2033-2037, 1978.
30. Stone. D. M., D. C. Stahl, G. R. Hansen and J. W. Gibb. The effects of 3,4 methylenedioxymethamphetamine (MDMA) and 3A met hy lenedioxyamphe tamine (MDA) on monoaminergic systems in the rat brain. EurJ Pharniacol 128: 41-48, 1986.
31. Stone, D. M., M. Johnson, G. R. Hanson and J. W. Gibb. A comparison of the neurotoxic potential of methylenedioxyamphetamine (MDA) and its N-methylated and N-ethylated derivatives. Eur J Pharmmol 134: 245-248, 1987.
32. Yeh, S. Y., G. Battaglia, E. O'Hearn, M. E. Molliver, M. J. Kuhar and E. B. De Souza. Effects of MDA and MDMA (ecstacy) on brain monoaminaergic systems: in vivo studies. Soc Neurosci A bstr 12: 1233, 1986.
Last Updated (Monday, 20 December 2010 19:52)












