3,4-Methylenedioxymethamphetamine and 3,4 Methylenedioxyamphetamine Destroy Serotonin Terminals in Rat Brain
Drug Abuse
3,4-Methylenedioxymethamphetamine and 3,4
Methylenedioxyamphetamine Destroy Serotonin Terminals in Rat Brain: Quantification of Neurodegeneration By Measurement of [³H]Paroxetine-Labeled Serotonin Uptake Sites'
GEORGE BATTAGLIA, S. Y. YEH, ELIZABETH O'HEARN, MARK E. MOLLIVER, MICHAEL J. KUHAR and ERROL B. DE SOUZA
Neuroscience Branch (G.B., S.Y.Y., M.J.K., E.B.D.S.), Addiction Research Center, National institute on Drug Abuse, Baltimore, Maryland and
Department of Neuroscience (E.O'H., M.E.M.), Johns Hopkins University, School of Medicine, Baltimore, Maryland
Accepted for publication May 15, 1987
ABSTRACT
This study examines the effects of repeated systemic adminis-tion (20 mg/kg s.c., twice daily for 4 days) of 3,4-methylene.xymethamphetamine (MDMA) and 3,4methylenedioxyamphetamine (MDA) on levels of brain monoamines, their metabolites and on the density of monoamine uptake sites in various regions of rat brain. Marked reductions (30-60%) in the concentration of 5-hydroxyindoleacetic acid were observed in cerebral cortex, hippocampus, striaturn, hypothalamus and midbrain at 2 weeks after a 4-day treatment regimen of MDMA or MDA; less consistent reductions in serotonin (5-HT) content were observed in these brain regions. In addition, both MDMA and MDA caused comparable and substantial reductions (50-75%) in the Pensity of ['Hlparoxetine-labeled 5-HT uptake sites in all brainregions examined. In contrast, neither MDMA nor MDA caused any widespread or long-term changes in the content of the catechotaminergic markers (i.e., norepinephrine, dopamine, 3,4 dihydroxyphenylacetic acid and homovanillic acid) or in the number of [3 HImazindol-labeled norepinephrine or dopamine uptake sites in the brain regions examined. These data demonstrate that MDMA and MDA cause long-lasting neurotoxic effects with respect to both the functional and structural integrity of serotonergic neurons in brain. Furthermore, our measurement of reductions in the density of 5-HT uptake sites provides a means for quantification of the neurodegenerative effects of MDMA and MDA on presynaptic 5-HT terminals.
MDMA and MDA are ring-substituted derivatives of methamphetamine and amphetamine, respectively. These compounds have been reported to exhibit both stimulatory and pychotomimetic properties (Anderson et al., 1978; Braun et al., '1980). Structural similarities between MDMA, MDA and their respective parent compounds suggest that these amphetaminelike compounds may exert effects on monoaminergic systems in brain comparable to those of the amphetamines. Various regimens of amphetamine and methamphetamine administration have been reported to significantly alter both DA and 5HT neurotransmission in brain in various species including rat, guinea pig and monkey (Hotchkiss and Gibb, 1980; Wagner et al., 1979, 1980; Ellison et al., 1978; Lorenz, 1981; Ricaurte et al., 1980; Sciden et al., 1975/76). The effects that have been observed include decreases in monoamine and monoamine metabolite levels, synthetic enzyme activities and decreases in the maximal activity of neuronal uptake systems.
In contrast to the reported effects of the amphetamines on both dopaminergic and serotonergic neurons, recent evidence suggests that their methylenedioxy derivatives, MDMA and MDA, may cause preferential long-terin alterations of serotonergic parameters in brain similar to those observed for the halogenated amphetamines (Fuller, 1978). Marked reductions in 5-HT content and in the maximal activity of 5-HT uptake systems have been observed in discrete regions of rat brain up to 14 days after repeated systemic administration of MDA (Ricaurte et al., 1985). In addition, both acute and short-term administration of MDMA or MDA have been shown to produce reductions in the content of 5-HT and 5-HIAA and decreases in tryptophan hydroxylase activity in various brain regions (Schmidt et al., 1986; Stone et al., 1986). Although these findings indicate that MDMA and MDA can significantly alter erotonergic transmission in brain, it is unclear whether the reductions in various parameters of serotonergic function(i.e., 5-11T and 5-HIAA content, tryptophan hydroxylase and 5-HT uptake activity) are the consequence of decreased functional activity in otherwise structurally intact neurons or are subsequent to the destruction of 5HT axons and/or terminals. Therefore, although MDMA and MDA may be considered neurotoxic with respect to their effects on 5-HT metabolic processes, additional measures are necessary to substantiate the neurodegenerative effects of these drugs on 5-HT neurons.
The present study was designed to assess and quantify the neurotoxic and neurodegenerative effects of short-term administration of MDMA and MDA on monoamine neurons in various regions of rat brain. Inasmuch as 5-11T uptake sites are highly concentrated on 5-HT-containing nerve terminals (Kuhar and Aghajanian, 1973), we can directly quantify the degree of n eu rodege ne ration of serotonergic terminals by measuring the reductions in uptake site density. We have reported recently the feasibility of using radioligand binding techniques and high affinity ligands; for 5-HT, DA and NE uptake sites to monitor the loss of monoamine terminals (Yeh et al., 1986). We report here that MDMA and MDA cause marked reductions in various markers (i.e., 5-HT, 5-HIAA and 5-HT uptake sites) of 5-HT terminals. Marked reductions in the density of 5-HT uptake sites in all brain regions examined which reflect the loss of 5HT axons and/or terminals indicate that both MDMA and MDA are potent, long-lasting and widely acting 5-HT neurotoxins.
Materials and Methods
Studies in this report were carried out in accordance with the Declaration of Helsinki and/or with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated li~ the National Institutes of Health.
Materials. ['H]Mazindol (19.7 Ci/mmol), ['Hjparoxetine (22.7 Ci/ mmol) and Formula 963 scintillation fluid were obtained from New England Nuclear (Boston, MA). MDA and MDMA were provided by the National Institute on Drug Abuse. Mazindol was supplied by Sandoz Pharmaceuticals (East Hanover, NJ) and citalopram was obtained from Lundbeck (Copenhagen, Denmark). All other compounds were obtained from Sigma Chemical Co. (St. Louis, MO).
Animal treatments. Male Sprague-Dawley rats (Harlan Industries, Indianapolis, IN), weighing 175 to 200 g, were housed under standard laboratory conditions (12-hr light/dark cycle) and had access to food and water ad libitum. Rats were injected s.c. twice a day, (approximately every 12 hr) with 20 mg/kg of MDA or MDMA (expressed as the free base) or saline vehicle (I ml/kg) for 4 consecutive days and then sacrificed 2 weeks after the last injection. This treatment regimen has been shown to produce maximal changes in brain 5-HT content after administration of MDA (Ricaurte et al., 1985). Cerebral cortex (i.e., frontoparietal cortex), hippocampus, striatum, hypothalamus and midbrain were dissected as described previously (Glowinski and Iverson, 1966), frozen immediately in liquid nitrogen then stored at -70*C until required for assay (less than 2 months).
HPLC determinations. Tissues from various brain regions were homogenized with 0A N HCIO, containing 0.1% cysteine. Catecholamines, 5-HT and their metabolites were measured with electrochemical detection after separation by reversed-phase HPLC. Samples were chromat.ographed on a Ci,, radial-pak cartridge and elated with a mobile phase at a flow rate of 0.9 ml/min. Each liter of the mobile phase contained 0.1 g of EDTA, 1.3 g of sodium heptyl sulfonate, 8 ml of triethylamineand 45 ml of acetonitrile and the p1l was adjusted to 2.85 with phosphoric acid (4.5 nil). Concentrations of catecholamines, 5HT and their metabolites in the brain tissues were calculated from standard curves, using DHBA as an int(mial sLandard. The standard curves were linear from 3 to 100 ng/ml (60-2000 pg/20 pl). The retention times for NE, DHBA, DA, DOPAC, 5-HIAA, HVA and 5HT were 3.15, 4.5, 6.46, 8.25, 13.52, 16.08 and 17.45 inin, respectively.
Measurement of uptake sites. Frozen brain regions front individual saline- and drug-treated rats were weighed and prepared as described previously (Habert et at., 1985). Tissues were placed in 50 volumes of ice-cold 50 mM Tris-HCI (pH 7A), 120 mM NaCl, 5 inNI KCI and homogenized using a Brinkman polytron (setting of 5, 2 X 30 se,c). The homogenate was centrifigued at 48,000 x g for 10 min with a subsequent resuspension and wash of the pellet. The final pellet was resuspended in the same buffer to a concentration of 15 mg of wet wt./ MI.
NE and DA uptake sites were measured using 6 nM ['Hlinazindol according to the method of Javitch et al. (1984). NE uptake site specific binding was defined as the difference between total binding and that in the presence of 0.3 uM DMI. DA uptake site specific binding was determined as the difference between the binding of ['111mazindol in the presence of 0.3,uM DMI and that in the presence of I uM mazindol. DMI at a concentration of 0.3 pM has been shown previously to selectively preclude the binding of ['H]mazindol to NE uptake sites without affecting binding to DA uptake sites. Assays were carried out in 0.5 ml of 50 mM Tris-HCI, 120 mM NaCl and 5 mM KC1 (pH 7.9 at 4'C). Tubes containing drugs and tissue (5-8 mg of wet wt./tube) were incubated on ice for 60 min then filtered rapidly over Whatman GF/C filters which had been presoaked in 0.05% PEI and washed with 15 ml of cold buffer.
[³H]Paroxetine binding to 5-HT uptake sites was carried out in the assay buffer described above with the following modifications of the original protocol (Habert et at., 1985). Increasing concentrations of I'Hjparoxetine (0.002-0.25 nM) in the absence and presence of I 'UM citalopram were incubated with 1.5 mg of tissue in 5 ml of buffer. Tubes were incubated for 2 hr at 22*C in order to ensure that the lowest concentrations of ligand had reached equilibrium. All tubes were then rapidly filtered over PEIpresoaked GF/C filters and washed with 15 ml of buffer. Filters were then equilibrated with 5 ml of Formula 963 scintillation fluid and counted at 54% efficiency in a Beckman 3801 scintillation spectrometer. For each concentration of ['Hlparoxetine, a parallel set of tubes was prepared with tissue and incubated similar to the experimental tubes. The tubes were centrifuged and an aliquot of the supernatant was taken in order to determine the "true" free 1'HJ paroxetine concentrations. Due to the high affinity (Kn = 10-20 pM) of this radioligand and consequent significant depletion which occurred at the lower concentrations, it was necessary to measure the actual amount of ['H]paroxetine at each of the concentration points. Proteins were determined according to the method of Lowry et al. (1951).
Data analyses. The data from the radioligand binding studies were analyzed by the computer program "EBDA" (McPherson, 1983) which provides initial estimates of equilibrium binding parameters by Scatchard and Hill analyses. All data were analyzed for statistical significance using one-way analysis of variance and Duncan's multiple range test.
Results
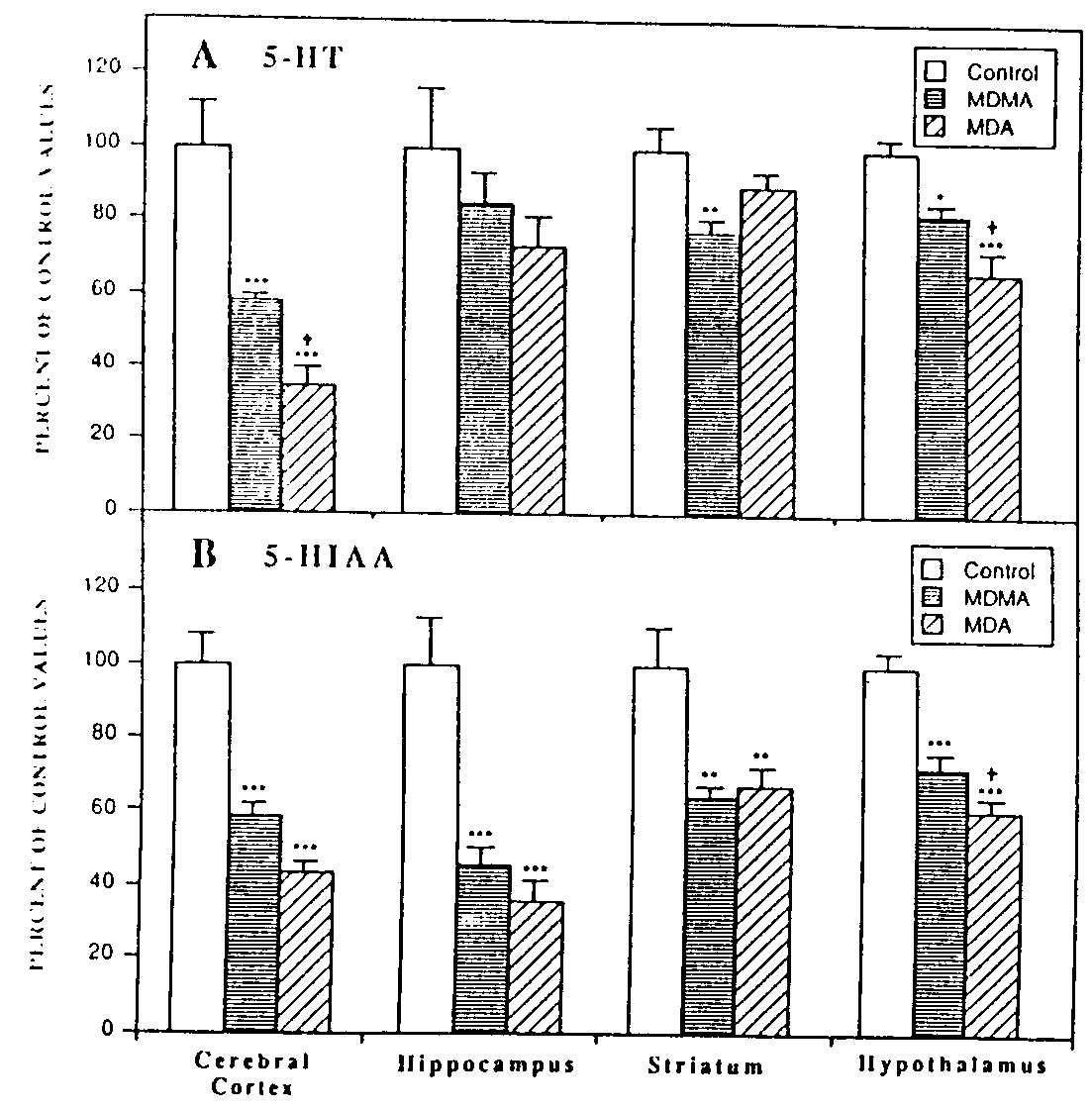
Fig 1 Effect of repeated systemic administration of MDMA and MDA on the concentration of (A) 5-HT and (B) its metabolite 5-HIAA in various brain regions. Rats were injected s.c. twice daily for 4 days with drug (20 mg/kg) or saline vehicle (1 mllkg) and sacrificed at 2 weeks after the last injection. 5-HT and 5-HIAA levels were measured using reversed phase HPLC as described under "Materials and Methods * " Data are plotted as a percentage of control values in each brain region and represent the mean and S.E.M. from four to six control and drug-treated rats. Control values for 5-HT and 5-HIAA in each of the regions were as follows: cerebral cortex, 504 ± 58 and 422 ± 32; hippocampus, 410 ± 67 and 684 ± 89; striaturn, 363 ± 22 and 492 ± 50; hypothalimus, 1605 ± 55 and 997 ± 42 pg/mg of tissue, respectively.
Monoamine and metabolite levels. The effects of repeated systemic administration of MDMA and MDA on brain monoamine and monamine metabolite levels were investigated at 2 weeks after the last injection. As shown in figure 1, A and B MDMA and MDA produced marked decreases in the content oi 5-HT and 5-HIAA in various brain regions. Both MDMA and MDA caused dramatic decreases in 5-HIAA levels in cerebral cortex, hippocampus, striaturn and hypothalamus (fig. 113). In hypothalamus, the reduction in 5-HIAA levels elicited by MDA was significantly greater (P <.05) than that observed with MDMA (fig. 113). When plotted as a percentage of control values in the respective brain regions, it was apparent that although decreases in 5-HIAA content were observed in all brain regions examined, the reductions in cerebral cortex and hippocampus (40-60%) were greater than those observed in striatum and hypothalamus (30-40%). With respect to 5-HT levels, marked decreases were observed in cerebral cortex and hypothalamus in both MDMA and MDA-treated rats (fig. 1A). Whereas small decreases were observed in hippocampal and striatal 5-HT content after either MDA or MDMA treatment, these reductions were found to be statistically significant only in- -aturn (P <.Ol) of MDMA-treated rats. When calculated as " jercentage of control 5-HT levels in the respective brain regions (fig. 1A), the data indicate a more marked reduction in cerebral cortex (40-60%) than in hypothalamus (18-33%).
In contrast with the marked and consistent effects of these compounds on serotonergic systems, neither MDMA nor MDA administration produced any widespread or consistent changes in levels of NE, DA, DOPAC or HVA in the various brain regions examined; however, small changes were observed in some brain regions. Both MDMA and MDA produced statistically significant increases in striatal DOPAC and cortical HVA content whereas only MDMA treatment resulted in an increase in hippocampal DOPAC levels (table 1).
Table 1
Effect of repeated systemic administration of MDMA and MDA on NE, DA and DA metabolite levels in various regions of rat brain
Regional brain levels of NE, DA, DOPAC and HVA in rats 2 weeks after administration of 20 mg/kg of MDMA or MDA. Drugs were administered s.c. every 12 hr for 4 consecutive days. Values (in picograms per milligram of tissue) represent the mean and S.E.M. of determinations in four to six individual rats. N.D., levels were below the sensitivity of the assay. Data were analyzed by one-way analysis of variance and Duncans multiple range test.
| Brain Region | NE | DA | DOPAC | HVA |
| |
||||
| Cerebral Cortex | ||||
| Control | 447 ± 53 | 59 ± 12 | 96 ± 14 | 19 ± 4 |
| MDMA | 424 ± 13 | 72 ± 3 | 73 ± 5 | 32 ± 4* |
| MDA | 404 ± 26 | 63 ± 4 | 94 ± 19 | 36 ± 5* |
| Hippocampus | ||||
| Control | 528 ± 62 | 31 ± 9 | 39 ± 6 | 6 ± 2 |
| MDMA | 573 ± 34 | 13 ± 5 | 65 ± 12* | 15 ± 5 |
| MDA | 608 ± 46 | 16 ± 4 | 32 ± 13 | 8 ± 5 |
| Striatum | ||||
| Control | N.D. | 6091 ± 596 | 3212 ± 159 | 788 ± 58 |
| MDMA | N.D. | 6974 ± 228 | 3954 ± 320* | 767 ± 46 |
| MDA | N.D. | 6168 ± 569 | 3669 ± 189* | 890 ± 48 |
| Hypothalamus | ||||
| Control | 3320 ± 209 | 569 ± 40 | 228 ± 43 | 54 ± 4 |
| MDMA | 3052 ± 159 | 457 ± 38 | 200 ± 30 | 54 ± 5 |
| MDA | 3577 ± 148 | 585 ± 67 | 229 ± 26 | 58 ± 3 |
* Significant difference from saline-treated control group at P < .05
Monoamine uptake site density. To further determine whether changes in 5-HT and/or 5-HIAA are the consequence of long-term suppression of serotonergic function in structurally intact neurons or whether MDMA and MDA may be affecting a neurodegenerative process, we measured the density of monciamine uptake sites in these brain regions to assess the structural integrity of neuronal processes. Both MDMA and MDA caused substantial reductions in the densities of 5-HT uptake sites in all brain regions examined. As shown in figure 2, the densities of 5-HT uptake sites are represented as a percentage of the respective control values in cerebral cortex, hippocampus, striatum, hypothalamus and midbrain. Significant reductions (all P < .001) were observed in cerebral cortex (60-70%), hippocampus (70-75%), striaturn (50%), hypothalamus (40-50%) and in midbrain (50-60%). Interestingly, MDA produced a significantly greater reduction in the density of 5HT uptake sites in cerebral cortex than that observed with MDMA. Scatchard analyses of ['Hiparoxetine saturation data in control- and drug-treated rats indicated that, in cerebral cortex, ['H]paroxetine binding was to a single population of sites (Hill coefficient values = 1.02, 1.01 and 1.03 in control, MDMA- and MDA-treated animals, respectively) and that there were no significant differences in the KD values between control- and drug-treated rats (18.8, 20.8 and 17.9 pM in control, MDMA- and MDA-treated rats, respectively).
In contrast with the effects of MDMA and MDA on the density of 5-HT uptake sites, neither MDMA nor MDA treatment caused any significant reduction in the levels of ['H] mazindol-labeled NE uptake sites in cerebral cortex, hippocampus or midbrain when compared with the respective salinetreated controls (fig. 3). Although a small reduction was noted in NE uptake sites in hippocampus this change was not statistically significant. Similarly, as shown in figure 4, no significant decreases were observed in the number of ['H] mazindol -labeled DA uptake sites in cerebral cortex, hippocampus, striaturn and midbrain after treatment with MDA. MDMA caused a statistically significant reduction (37%) in the density of DA uptake sites only in the midbrain. These findings are consistent with the results from our measurements of the content of catecholamines and catecholamine metabolites in various brain regions and support the contention that neither MDMA nor MDA cause any marked widespread alterations in the integrity of catecholaminergic neurons.
Fig. 2. Effect of repeated systemic administration of MDMA and MDA on the density of 5-HT uptake sites in various brain regions. Rats were injected s.c. twice daily for 4 days with MDMA or MDA (20 mg/kg) or saline vehicle (1 ml/kg) and sacrificed at 2 weeks after the last injection. Values were determined from saturation studies in each of the regions except striaturn and hypothalamus in which the density of 5-HT uptake sites was assessed using a saturating concentration (0.25 nM) of [3 Hlparoxetine. No significant differences from control KI) values (10-20 pM) were observed in either MDMA- or MDA-treated rats. Data are plotted as a percentage of the 5-HT uptake site density observed in controls in each brain region and represent the mean and S.E.M. from three to six rats per group. Control values were as follows: cerebral cortex, 338 ± 10, hippocampus, 360 ± 17; striaturn, 344 ± 30; hypothalamus, 775 ± 36; and midbrain, 570 ± 16 fmol/mg of protein. Data were analyzed by one-way analysis of variance and Duncans multiple range test. Significant differences at P < .001 from control values are denoted by***, whereas differences at P < .001 between MDA and MDMA treatments are denoted by t.
| Fig. 3. Effect of repeated systemic administration of MDMA and MDA on the density of NE uptake sites in various brain regions. Rats were injected s.c. twice daily for 4 days with MDMA or MDA (20 mglkg) or saline vehicle (1 milkg) and sacrificed at 2 weeks after the last injection. NE uptake sites were measured using 6 nM 13 Hlmazindol in the presence of selective blockers as described under Waterials and Methods." Data are plotted as a percentage of control values in each brain region and represent the mean and S.E.M. from six control, MDMA- and MDAtreated animals. Control values of NE uptake sites were as follows: cerebral cortex, 164 ± 6; hippocampus, 176 ± 9; midbrain, 157 ± 13 fm61Img of protein. | Fig. 4. Effect of repeated systemic administration of MDMA and MDA on the density of DA uptake sites in various brain regions. Rats were injected s.c. twice daily for 4 days with MDMA or 1ADA (20 mglkg) or saline vehicle (1 milkg) and sacrificed at 2 weeks after the last injection, DA uptake site specific binding was determined using 6 nM [31-11mazindol in the presence of selective blockers as described under Waterials and Methods." Data are plotted as a percentage of control values in each brain region and represent the mean and S.E.M. from six control, MDMAand MDA-treated rats. Control values were: cerebral cortex, 45 ± 7; hippocampus, 28 ± 6; striatum, 342 ± 26; micibrain, 40 ± 3 fmollmg of protein. |
Discussion
The present study demonstrates that short-term administration of NIDMA or MDA for 4 days results in profound reductions in serotonergic markers in rat brain. Decreases in 5-HT and 5HIAA content were observed in various brain regions for up to 2 weeks after drug administration. In contrast, MDMA and MDA produced only minor changes in catecholamine markers. These data are consistent with other reports demonstrating that MDMA and MDA primarily alter serotonergic function in brain (Ricaurte et al., 1985; Schmidt et al., 1986; Stone et al., 1986), The decreases in 5-HT and 5-HIAA content after short-term administration of MDMA and MDA may result in part from the previously reported effects of these compounds on inhibition of tryptophan hydroxylase activity (Stone et al., 1986) and/or their ability to increase 5-HT release (Nichols et al., 1982; Johnson et al., 1986). However, marked decreases in 5-HT, 5-HIAA and tryptophan hydroxylase activity also have been reported after single injections of lVIDMA or MDA (Schmidt et al., 1986; Stone et al., 1986; Ricaurte et al., 1985). Although the changes in the serotonergic markers described above after short-term administration of MDMA and MDA suggest neurotoxic actions of these drugs on serotonergic neurons in brain, it is unclear whether these neurotoxic effects represent suppressed serotonergic neurotransmission in structurally intact neurons or whether the reductions in these markers reflect neurodegenerative processes. The neurode generative effects of MDA on brain 5-HT terminals were postulated recently based on decreased 5-HT levels in conjunction with histologic evidence of terminal degeneration in striaturn (Ricaurte et al., 1985). Although the data of Ricaurte et al. (1985) provide strong circumstantial evidence for the selective destruction of 5-HT neurons after MDA administration, degeneration was assessed in only one brain region using the nonspecific Fink-Heimer method which stains for the dege ration o various types of terminals (Fink and Heimer, 1967). Therefore, the specific loss of 5-HT terminals as well as the extent of damage to 5-HT terminals throughout the brain caused by MDMA or MDA remains to be directly demonstrated. Inasmuch as 5-HT uptake sites are highly concentrated on 5-HT-containing nerve terminals (Kuhar and Aghajanian, 1973), a more specific means of assessing and quantifying a loss of 5-HT terminals would be to measure changes in the density of 5-HT uptake sites. These sites can be selectively labeled using specific probes such as ['1-11paroxetine (Habert et j al., 1985). As shown in figure 2, both MDMA and MDA cause comparable and marked reductions in the density of 5-HT uptake sites in discrete regions of rat brain. Our contention that these changes represent the neurodegenerative effects of these drugs on brain 5-HT terminals is supported by our preliminary immunocytochemical data demonstrating destruction of 5-HT axons and terminals in rat brain (O'Hearn et al., 1986). The marked reductions in 5-HT uptake sites in all the brain regions examined in the present study would suggest that I both MDMA and MDA exhibit little regional specificity in their ability to destroy brain 5-HT terminals. In order to assess further the regional specificity of the neurodegenerative effects of these compounds on serotonergic terminals, we have visualized changes in 5-HT uptake sites at the light microscopic level after in vitro autoradiography.
Although preliminary data from autoradiographic studies confirm the drastic reductions in 5-HT uptake sites in the brain regions used in the present study, MDMA and MDA did not produce any marked change in the density of 5-HT uptake sites in certain brain regions such as the lateral septum and midbrain raphe nuclei (Battaglia et al., 1987). As there is a substantial serotonergic innervation of midbrain nuclei such as the periaqueductal gray and substantia nigra (Steinbusch, 1983), decreases in the density of 5-HT uptake sites in midbrain seen in the present study probably represents the loss of ser*otonergic projections to midbrain nuclei rather than the destruction of perikarya in the raphe. Thus, there may be some neuroanatomical and regional specificity to the neurodegenerative effects of these drugs on brain 5-HT systems.
Some subtle differences in the effects of MDMA and MDA on serotonergic parameters in different regions of brain have been observed in the present study. For example, both MDA and MDMA produce significant decreases in the concentration of 5-HT in cerebral cortex and hypothalamus with little or no effect in hippocampus and striaturn. Reductions in the density of .5-HT uptake sites in all the brain regions examined are i Alleled more closely by similar but somewhat smaller reductions of 5-HIAA. These data suggest that using 5-HT concentrations as an index of degeneration of 5-HT terminals might underestimate the actual magnitude of the neurodegenerative effect. The large disparity in correspondence between reductions in 5-HT concentrations and the density of 5-HT uptake sites in specific brain regions (e.g., hippocampus and striatum) that we have observed after MDMA and MDA treatment may be explained, in part, by the increased accumulation of 5-HT that occurs in axons of passage secondary to 5-HT terminal degeneration (O'Hearn et al., 1986). Similar morphologic changes have been reported previously in serotonergic fibers after administration of 5,6- or 5,7-dihydroxytryptamine (Baumgarten et al., 1973; Bjorklund et al., 1973). Sensitivity differences in various brain regions to the effects of MDMA and MDA are not unique as previous data have demonstrated differential sensitivity to the effects of methamphetamine (Ricaurte et al., 1980) and PCA (Kohler et al., 1978; Fulleret at., 1975) on serotonergic systems in various brain regions. Biochemical and histochemical data suggest that PCA primarily affects the ascending 5-HT systems whereas the descending pathways are left intact (Kohler et al., 1978; Fuller, 1978).
The neurotoxic effects of MDMA and NIDA appear to be exerted preferentially on serotonergic neurons its no widespread changes in a variety of catecholamine markers were seen after chronic administration of these drugs. Specifically, no significant alteration in NE or DA concentrations were seen after administration of MDNIA or MDA in any of the brain regions examined; the effects of MDA and MDMA administration on the concentrations of DOPAC and HVA were more variable. Small but significant increases in DOPAC and/or HVA were seen in cerebral cortex, hippocampus and striaturn after chronic administration of these methylenedioxyamphet.ainine derivatives. Similar increases in DA metabolite levels, reflecting increases in DA turnover, have been observed in discrete regions of brain after both acute (Schmidt et al., 1986) and chronic (Stone et al., 1986) administration of MDMA and MDA. Because 5-HT-containing terminals are present in high concentrations in midbrain areas (substantia nigra and ventral tegmental area) containing DA cell bodies (Steinbusch, 1983), tile degeneration of 5-HT terminals in these regions after MDMA or MDA treatment may be responsible for the observed changes in DA metabolites. Despite these small effects on DA turnover, MDMA and MDA do not appear to produce any widespread destruction of catecholaminergic terminals as the only change observed was a small reduction in DA uptake sites in midbrain after administration of MDMA. The reasons for the decrease in DA uptake sites are at present unclear. Preliminary immunocytochemical data indicate that there are no changes in the density or morphology of catecholarnine axons after chronic administration of MDMA or MDA (O'Hearn et al., 1986). We are presently carrying out detailed autoradiographic studies to identify any discretely localized small change in catecholaminergic neurons.
In summary, the data we present here demonstrate that repeated, systemic administration of either MDMA or MDA can cause profound and preferential destruction of 5-HT terminals in various regions of rat brain. The use of in vitro radioligand binding techniques to measure the density of monoamine uptake sites provides a means for quantification of the extent of degeneration of 5-HT terminals. This approach in conjunction with "conventional" measures of reductions in monoamine and monoamine metabolite levels allow us to conclude that whereas MDA and MDMA are without any widespread long-term effects on the functional and structural integrity of catecholamine neurons, these compounds cause a longlasting degeneration of 5-HT neurons in a number of regions of rat brain. The physiological consequences and compensatory mechanisms in response to the long-term destruction of serotonergic neurons in brain remain to be assessed.
ABBREVIATIONS: MDMA, 3,4-methylenedioxymethamphetamine; MDA, 3,4-methylenedioxyamphetamine; DA, dopamine; 5-HT, serotonin; 5-HIAA,
5-hydroxyindoleacetic acid; NE, norepinephrine; HPLC, high-performance liquid chromatography; DHBA, dihydroxybenzyiamine; DOPAC, 3,4
dihydroxy phenyl acetic acid; WA, homovanillic acid; DMI, desipramly~l~". -chioroamphetamine.
Acknowledgments
The authors thank Brian Kuyatt for excellent technical assistance, Drs. Charles Haertzen and Lawrence Thompson for help with data analysis andTerrie Pierce and Mary Flutka for manuscript preparation.
References
ANDERSON, G. M_ BRAUN, G., BRAUN, U., NICHOLS, D. E. AND SHULGAN, AT.: Absolute Confisguration and Psychotomimetic Activity, NIDA Research Monograph vol. 22, pp. 8-15, 1978.
BATFAGLIA, G_ KU11AR, M. J. AND DE SOUZA, E. B.: MDMA-iiidticed neurotoxicily visualized by in vitro autoradiography: Differential sensitivity of serotonergic neurons. Fed. Proc. 46: 404, 1987.
BAUMCARTEN, 11. Gi., BJORKLUND, A,, LACI1ENMAYER, L. AW) NOBIN, A.: Evaluation of (lie effects of 5,7-dihydroxytryptarnine on serotonin and catecholamine neurons in the rat CNS- Acta Physiol. Scan (suppl.) 391: 1-19, 1973.
BJORKLUNI), A_ NOBIN, A. AND STEVENI, IL: Regeneration of central serotonin neurons after axonal degeneration induced by 5,6-dihydrox~ltryptarnine. Brain Res 50: 214-220, 1973.
BRAUN, U., SHULGIN, A. T. AND BRAUN, G.: Centrally active ri-substituted analogs of 3.4-rnethyleric(lioxy I)Iienylisol)rc)l)ylamine (3,4.Methyl.no'lio.y.m phetarnine). J. Pharm. Sci. 69: 192-195, 1980.
FLIASON, G_ FISON, M. S., HUBERMAN, M. S. AND DANIEL, F.: Long-tern changes in cloparnine innervation of caudate nucleus after continuous arriphet amine administration. Science (Wash. D0 201: 276-278, 1978.
FINK, R. P. AND HEIMER, L: Two methods for selective silver impregnation of degenerating axons and their synaptic endings in the central nervous systern Brain Res. 4: 369-374, 19(17.R. W.: Neurochernical effects ofserotonin neurotoxins. Ann. N.Y. Acad. Sci. 305: 178-181, 1978.
Ftji,i,~,R, R. W_. PERRY,K. W. AND M0i,i.ov, B. B.: Reversible and irreversible phases of serotonin depletion by 4-chloroamphetarnine. Eur. J. Pharmacol. 33: 119-124, 197.5.
GLOWINSKI, J. AND IVERSON, L. L: Regional studies of catecholamines in rat brain. 1. The disposition of 3 H^norephinephrine, 'H-dopamine and 311-DOPA in various regions of the brain. J. Neurochem. 13: 665-669, 1966.
HABERT, E., CRAHAM, D., TAI1RA0UJ, 1--- CLAUSTRE, Y. AND LANGER, S. Z.: Characterization of '11-paroxetine binding to rat cortical membranes. Eur. J. Pharmacol. 1 IS: 107-114, 1985.
HOTC11Kiss, A. J. ANO GIBB, J. W.: Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. 3. Pharmacol. Exp. Ther. 214: 257262, 1980.
,vi,i,cii, 3. A_ BLAUSTEMN, R. 0. AND SNYDER, S. H.: ['H1Mazindol binding associated with neuronal clopainine and norepinephrine uptake sites. Mol. Pharmacol. 26: 35-44, 1984.
JOHNSON, M. P.. 11OFFIVIAN, A-1. ANi) Nicitoi.s, D. E.: Effects ofthe emintiomers of MDA, MDMA and related analogues on ['Hiserotonin and ['Hldol)amine release from superfused rat brain slices. Eur. J. Pharmacol. 132: 269- 276, 1986.
KoliLF,it, C., ROSS, S. Il., SzItLI110, 11. AND OGREN, S . 0-: Long-term biochemical effects ofp-chloroamphetamine in the rat. Ann. N.Y. Acad. Sci. 305: 645-663, 1978. 9
KU11AR, M. J. AND AcIMAJANIAN, G. K.: Selective accumulation ol 'Fl-serotonin by nerve terminals of raphe neurons: An autoradiographic study. Nature (Lond.) 241: 187-189, 1973.
LORENZ, H.: Fluorescence histochemistry indicates damage of striatal dopamine Vol. 242 nerve terminals in rats after multiple doses of methamphetaniine. Life Sci. 28: 911-916,1981.
LowRY, 0. If., RosEiiRouc.ii, N. J_ FARR, A. L. AND RANDALL, It. J.: Protein measurement with the Folin phenol reagent. 3. Biol. Cliern. 193: 265-275, 1951.
M&HERSON, G. A.: A practical computer-based approach to the analysis of radioligand binding experiments. Comput. Programs Biomed. 17: 107-114, 19&3.
ICHOLS, D. E., LLOYD, D. H_ HOFFIV[AN, A. J., Niciioi.s, M. B. AND YIM, G K.: Effect of certain hallucinogenic amphetamines analogues on the release of 'H-serotonin from rat brain synaptosomes. J. Med. Chem. 25; 530-535, 1982.
4* O'liKARN, E., BATTAGLIA, C, Dm SOUZA, E. B., KUMAR, M. J~ AND M1,1.1VE1t,M_ E.: Systemic MDA and MDMA, psychotropic substituted amphetamines, produce serotonin neurotoxicity. Soc. Neurosci. Abstr. 12: 1233, 1986.
RICAURTE, G., BRYAN, G., STRAUSS, L, SEIDEN, L. AND SCHUSTEit, C.: Hallucinogenic amphetamine selectively destroys brain serotonin nerve terminals. Science (Wash. DC) 229: 986-988, 1985.
RICAURTE, G. A., SCHUSTER, C. R. AND SEIDEN, L S.: Lcing term effeCts of repeated methylamphetarnine administration on dopamine and serolonin neurons in the rat brain: A regional study. Brain Res. 193: 153-163, 1980.
#SCHMIDT, C. J., WU, L. AND LoVENBERG, W.: Methyl enedioxya m p hetain i lie: A potentially neurotoxic amphetamine analogue. Eur. J. Pharmacol. 124: 175178, 1986.
SEIDEN, L S., F1scilMAN, M. W, AND SCRIUSTER, C. R : Long-term inetham. phetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Dnig Alcohol Depend- 1: 215-219, 1975/76.
STEINBUSH, H. W. M.: Serotonin-immunoreactive neurons and their projections. in the CNS. In Handbook of Chemical Neuroanatorny, vol. 3, ed. by A. Bjorklund,T. Hokfelt and.'~f-.1. Kuhar,pp. 68-125, Elsevier Science Publishers, New York, NY, 1983.
*STONE, D. M., STAim, D. C., HANSEN, G ---It. AND CIBB, J. W.: The effects of 3,4 met hylenedioxyinethampheta m i ne (MDMA) and 3,4 methylenedioxyamphetamine (MDA) on monoarninergic systems in the rat brain. Eur. J. Pharmacol. 128: 41-48, 1986.
WAGNER, G. C., RICAURTE, G. A_ SEMEN, L. S., SCRUISTER, C. R., MILLER, H. J. AND WESTLEY, J.: Long lasting depletions of striatal dopamine and the loss of dopamine uptake sites following repeated administration of methampheldmine. Brain Res. 181: 151-160, 1980.
WAGNER, G. C., SEIDEN, L. S. AND SCHUSTER, C. R. Methamphetamine-induced changes in brain catecholamines in rats and guinea pigs. Drug Alcohol Depend. 4: 435-438, 1979.
YEll S. Y., BATTAGLIA, (,., O'llEARN, E., Moi.i.ivF.it, M. E., KIMAR, M. J. AND DE SouzA, E. B.: Effects of NIDA and MDMA (cestit..s~y) on brain nionoanlinergic systems: In vivo studies. Soc. Neurosci. Abstr. 12: 1233, 1986.
Send reprint requests to: Errol B. De Souza, Ph.D., Neuroscience Branch, National Institute on Drug Abuse/Addiction Research Center, P.O. Box 5180,
Baltimore, MD 21224.
Last Updated (Monday, 20 December 2010 19:47)












