Lasting Effects of (±)-3,4-Methylenedioxymethamphetamine (MDMA) on Central Serotonergic Neurons in Nonhuman -Primates: Neurochemical Observations'
Drug Abuse
first published in the journal of pharmacology and experimental therapeutics vol 261 no2
Lasting Effects of (±)-3,4-Methylenedioxymethamphetamine (MDMA) on Central Serotonergic Neurons in Nonhuman -Primates: Neurochemical Observations'
GEORGE A. RICAURTE, ANNE L. MARTELLO, JONATHAN L. KATZ and MARY B. MARTELLO
Department of Neurology, Francis Scott Key Medical Center, Johns Hopkins University School of Medicine, Baltimore, Maryland (A.L.M., M.B.M.,
G.A.R.) and the Addiction Research Center, National Institute of Drug Abuse, Baltimore, Maryland (J.L.K.)
Accepted for publication February 6, 1992, 'Support for this study was provided by the National Institute on Drug Abuse (Grant DA 05707 to G.A.Rj. )
ABBREVIATIONS: MDMA, (±)-3,4-methylenedioxymethamphetamine; MDA, (±y3,4-Methylenedioxyamphetamine; 5-HT, serotonin; 5-HIAA, 5hydroxyindoleacetic acid; DOPAC, dihydroxyphenylacetic acid; DA, dopamine; NE, norepinephrine; FC, frontal cortex; PFC, prefrontall cortex; OC, occipital cortex; TC, temporal cortex; SC, sornatosensory cortex; HYP, hypothalamus; TH, thalamus; HI, hippocampus; CH, caudate nucleus head; CB, Caudate nucleus body, PR. putamen rostral; PC, putamen caudal; HPLC, high-performance liquid chromatography; ANOVA, analysis of variance.
ABSTRACT
The purpose of this study was to assess the duration of (±)-3,4methylenedioxymethamphetamine's (MDMA's) effects on sero,gnin containing neurons in nonhuman primates. Fifteen squirrel .nonkeys were used: three served as controls, 12 received MIDMA s.c. at a dose of 5 mglkg twice daily for 4 consecutive days. Two weeks, 10 weeks, 8 months and 18 months after drug treatment, groups (n = 3) of MDMA-treated monkeys, along with controls, were examined for regional brain content of serotonin and 5-hydroxyindoleacetic acid, and for the number of [3 H] paroxetine-labeled serotonin uptake sites. Two weeks after MDMA treatment, monkeys showed profound reductions in all three serotonergic presynaptic markers. By 10 weeks, there was evidence of partial recovery in some brain regions (e.g, hippocampus. caudate nucleus, frontal cortex). However, by 18 months, it was evident that recovery did not continue, as serotonergic deficits returned to the level of severity observed 2 weeks after MDMA treatment. This was the case in all brain regions examined except the thalamus and hypothalamus. In the thalamus, the level of serotonin increased to 63% of control, whereas that of 5hydroxyindoleacetic acid recovered completely. In the hypothalamus, concentrations of serotonin and 5hydroxyindoleacetic acid were 140 and 187% of control, respectively. These results suggest that MDMA produces lasting effects on serotonergic neurons in nonhuman primates, with most brain regions showing evidence of persistent denervation and some showing signs of reinnervation (thalamus) or possibly even hyperinnervation (hypothalamus). The morphological and functional correlates of these enduring neurochernical changes in the MDMA-treated primate remain to be delineated.
In recent years, MDMA has emerged as a recreational drug of abuse (Bost, 1988). Although the toxicity of MDMA toward 6-HT-containing neurons in rodents (Stone et aL, 1986; Schmidt, 1987; Commins et aL,1987; Battaglia et al., 1987; Mearn et al., 1988) as well as in nonhuman primates (Ricaurte et al., 1988a; Insel et aL, 1989; Kleven et aL, 1989; Wilson et al., 1989) is now well established, the fate of damaged serotonergic neurons remains uncertain. In particular, it is not known if all serotonergic neurons damaged by MDMA recover and, if they do, whether they reestablish normal synaptic contacts.
Two reports have suggested that at least in rodents, substantial serotonergic recovery does take place after MDMA injury. The first, by Battaglia et al. (1988), shows that although MI)MA-treated rats sustain an initial severe loss of 5-HT content and uptake, I year later they have a normal number of ['111paroxetine-labeled 5-HT uptake sites (though 5-HT remains depleted by approximately 40%). The second, a report based on a series of immilnohistochemical studies (Molliver et al., 1990), shows that recovery of presynaptic 5-HT markers is most likely related to regrowth of serotonergic axons. Interestingly, axonal reinnervation in these studies was found to involve fine serotonergic axons (Wilson and Molliver, 1990; Molliver et al., 1989), which are selectively damaged by UDA and MDMA (O'Hearn et al., 1988; Wilson et aL, 1989). Thick beaded axons, which are spared by MDMA (O'Hearn et aL, 1988; Wilson et al., 1989), showed no evidence of collateral sprouting (Molliver et aL, 1990; Wilson and Molliver, 1990). On the basis of these findings, Molliver and colleagues (1990) have concluded that although further studies are needed to determine if normal reinnervation patterns are established, MI)MA-treated rats show evidence of 5-HT axonal regeneration.
Whether similar axonal regeneration takes place in the MDMA-treated primate is not known, but important to determine, because findings in nonhuman primates could be more indicative of what is likely to occur in humans. In two respects, nonhuman primates are already known to differ from rodents in their response to MDMA: 1) monkeys are more sensitive than rats to the serotonin- depleting effects of the drug (Ricaurte et al., 1988a; Ricaurte, 1989) and 2) neuronal perikarya in the dorsal raphe nucleus of the monkey develop pathological changes (inclusions) (Ricaurte et al., 1988a), whereas those in the rat appear entirely unaffected (Molliver et al., 1990). Based on these differences, the long-term effects of MDMA in rodents and primates might be expected to differ. In particular, it might be that although the effects of MDMA in the rodent are reversible, those in the primate are permanent. To test this hypothesis, the present study was undertaken.
Methods
Animals. Adult squirrel monkeys (Saimiri sciureus) of both sexes were used. Their weight ranged from 499 to 906 g. The precise age of the monkeys could not be determined with certainty because the animals were feral reared. However, neither extremely young nor old animals were used. Monkeys were housed individually in standard steel -ages with free access to food (Purina Monkey Chow) and water ,aroughout. The colony room was maintained at 22-24*C. Fluorescent lighting in the room was automatically turned on daily at 6:00 A.M. and off at 6:00 P.M.
MDMA treatment. Racemic MDMA, as the hydrochloride salt, was injected at a dose of 5 mg/kg twice daily (at approximately 8:00 A.M. and 5:00 P.M.) for 4 consecutive days. This dosing regimen of MDMA was selected because it produces a degree of axonal damage in the squirrel monkey (Ricaurte et aL, 1988; Wilson et al., 1989), which is comparable to that from which rats have been reported to recover (Battaglia et al., 1989a; Molliver et aL, 1990). MDMA wasmdissolved in a sterile 0.9% sodium chloride solution and administered s.c. on an ml/ kg basis, with the dose expressed as the salt. Animals tolerated MDMA treatment without apparent difficulty.
Brain dissection. The brain was removed from the skull and placed on its ventral surface on a Petri dish over ice. Tissue samples were then obtained from the following cortical regions bilaterally. FC, OC, TC and SC. In all cases, care was taken to remove white matter underlying the cortical tissue. The HYP was dissected free using the optic chiasm, infundibulum and oculomotor nerves as landmarks. The hypothalamic sample was isolated by trimming away the optic tracts and then removing a diamond-shaped tissue sample that had the tuber cinereum at its center. The brain stem was obtained by making a coronal cut from the rostral border of the basis*pedunculi to the anterior margin of the superior colliculi. At this point, the cerebral hemispheres were separated by sectioning the corpus callosurn. The HI was removed by peeling it away from the temporal lobe and sectioning its point of attachment (subiculum). The TH was dissected using the hypothalamic sulcus and lateral and third ventricles as landmarks. Coronal sections of each hemisphere were then made with the ventral surface facing upwards. Proceeding caudally from the FC, six coronal slices were made. All slices were approximately 2 to 3 mm in thickness. From the first three coronal slices, FC tissue was obtained and designated FC1. The fourth slice was obtained by making a coronal cut at the point were the olfactory tracts begin to diverge laterally (approximately 2 to 3 mm anterior to the optic chiasm). From this section, additional FC tissue was collected from that portion of the cortical mantle overlying the CH. This FC tissue was designated FC2. The CH was also obtained from this section. The fifth coronal slice was made by making a cut anterior to the optic chiasm, and another posterior to the marnmillary bodies. From this slice, additional FC3 tissue overlying the CB was obtained, along with tissue of the caudate nucleus (CB1) and putarnen (PR). From the sixth and final coronal slice, additional tissue from the CB (CB2) was obtained, along with an additional sample of the putamen (PC). Once dissected free, tissue samples were immediately wrapped in aluminum foil and stored frozen in liquid nitrogen until assay.
Measurement of brain monoamines and their major metabolites. Concentrations of 5-HT, DA, NE, 5-HIAA and DOPAC in brain tissue were measured by reverse phase liquid chromatography (HPLC) coupled with electrochemical dewtion using the method of Kotake et al. (1985) with minor modification. Briefly, frozen tissue was weighed, placed in 10 parts ice-cold 0.4 N perchloric acid, then homogenized for 15 see using a Polytron homogenizer (setting = 5). The homogenate was centrifuged for 10 min at 15,000 rpm in a refrigerated Sorvall RC2B centrifuge, and fractions of the supernatant were transferred to polypropylene tubes, which were then returned to liquid nitrogen until assay. Separation of monoamines and their metabolites was accomplished with a Brownlee Spheri-5 RP-18 250 X 4.6 mm column (5-yin particle size). For separating the various monoamines and their metabolites, a mobile phase consisting of 98 parts of an aqueous phase (125 mM citric acid, 125 mM sodium phosphate, 0.27 mM EDTA and 0.12 mM sodium octane sulfate) and 2 parts methanol with a pH of 2.5 was used. For separating NE, a mobile phase consisting of an aqueous phase (0.09 M sodium acetate, 0.035 M citric acid, 0.13 mM EDTA and 0.23 mM sodium octane sulfate) with a pH of 4.6 was used (Kilpatrick et al., 1986). The flow rate of the mobile phase was approximately 1.0 ml/ min. The column was maintained in a CTO-6A column oven module (Shimadzu) at 40*C. Detection was achieved by means of an amperometric L-ECD6A detector (Shimadzu, Columbia, MD), with a glassy carbon working electrode and a silver/silver chloride reference electrode. The fixed potential difference between the reference and working electrodes was +0.70 V. The electrochemical response was quantified using a Shimadzu Chromatopac C-114A data processor equipped to measure the area under the curve for a given sample and compare it to that of standards processed in an identical manner. The sensitivity of this method is approximately 20 pg per 20 1A of sample. Samples from the various treatment groups were always assayed in parallel.
Measurement of serotonin uptake sites. ['H]Paroxetine-labeled 5-HT uptake sites were measured using the method of Habert et al. (1985), as modified by Battaglia etat. (1987). Briefly, frozen brain regions were weighed, placed in 50 volumes of ice-cold 50 mM TrisHC1 (pH 7.4) buffer containing 120 mM NaCl and 5 mM KCI and homogenized for 30 see with a Brinkman Polytron homogenizer. The homogenate was centrifuged at 19,500 rpm for 10 min. The pellet was resuspended in the same buffer and centrifuged once again as above. The final pellet was resuspended in the same buffer at a concentration of 6 mg/ml. Diluted aliquots (0.5 ml) of the tissue suspension containing 3 mg of tissue were then placed in tubes. To these tubes, increasing concentrations (0.013-0.207 nM) of '[H]paroxetine (20.5 Ci/mM, New England Nuclear, Boston, MA) were added. This was done in both the presence and absence of 1 uM citalopram. Tubes were then incubated for 1 hr at 22 ± I*C. After incubation, the tubes were rapidly filtered through GFB (Whatman) filters. The filters were washed three times with 5 ml of buffer, and transferred to plastic vials containing 10 ml of scintillation cocktail. Radioactivity was counted in a Beckman scintillation spectrometer at an efficiency of 64%. Specific binding was calculated by subtracting the counts obtained in samples containing citalopram (nonspecific binding) from the counts obtained in identical samples not containing citalopram (total binding). Assays were performed in triplicate. Specific ['111paroxetine binding was expressed as pM ['Hlparoxetine bound/mg tissue. Binding data were analyzed with the aid of the computer program entitled EBDA (GraphPAD Software, San Diego, CA). As with HPLC monoamine determinations, samples from the various treatment groups were always assayed in parallel.
Statistical analysis. The significance of differences among group means was evaluated by means of analysis of variance (ANOVA). Once a significant F test was obtained, Duncan's multiple range comparisons were performed to assess the significance of differences among various treatment groups. The a level for all post hoc comparisons was .05. Version 4.1 of the computer program STATPAK (Northwest Analytical Inc., Portland, Oregon) was used.
Drugs and chemicals. MDMA hydrochloride was obtained from the National Institute on Drug Abuse. 5-HT creatinine sulfate, DA uptake sites (B...), without causing a significant change in the hydrochloride, NE hydrochloride, 5-HIAA and DOPAC were purchased apparent affinity constant (Kd) of the residual sites (fig. 2). from the Sigma Chemical Co. (St. Louis, MO). Tritiated paroxetine Reductions in 5-HT content, ['Hjparoxetine binding and 5 (specific activity 20.5 Ci/mM) was purchased from New England Nuclear Co.
Results
Serotonergic Neurons
Effects at 2 weeks. Two weeks after MDMA treatment (5 mg/kg, s.c., twice daily for 4 days), there was a marked depletion of 5-HT in all brain regions examined (fig. 1A). MDMA treatment also produced a large depletion of 5-HIAA (fig. 1B). Scatchard analysis of ['H]paroxetine binding showed that along with depletions of 5-HT and 5-HIAA, MDMA treatment reduced the maximal number of ['Hlparoxetine-labeled serotonin uptake sites (ßmax) without causing a significant change in the apparent affinity constant K4 of the residual sites (fig 2). reduction in 5-HT content, 3H parotexine binding and 5-HIAA concentration were comparable in magnitude (table 1).
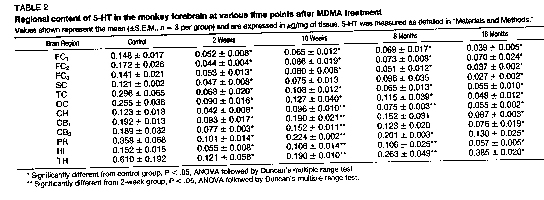
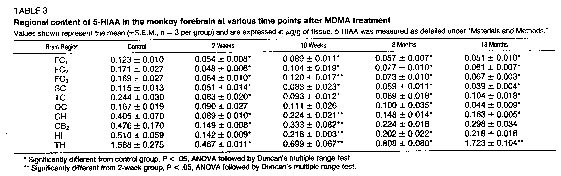
Time course. By 10 weeks, a trend toward recovery of 5HT (table 2), 5-HIAA (table 3) and [3 H]paroxetine binding (fig. 3, A-C) was evident in some (but not all) of the forebrain regions examined. For example, in the C11 and HI, there was indication that all three presynaptic markers had partially recovered by 10 weeks. However, in other brain regions (e.g., FC3), only recovery of 5-HIAA achieved statistical significance (tables 2 and 3 and fig. 3B). Evidence of partial recovery was still apparent 8 months after MDMA treatment in some brain regions (e.g., HI). However, by 18 months, it was clear that recovery did not continue in any of the brain regions examined except for the TH (tables 2 and 3) and HYP (fig. 4).
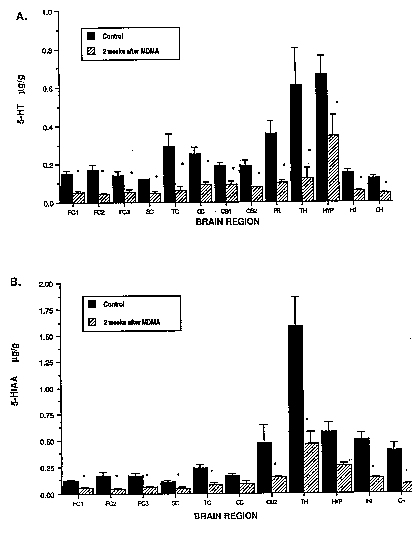 |
Fig. 1. Depletion of A) 5-HT and 13) 5-HIAA in various regions of the monkey forebrain 2 weeks after MDMA treatment. MDMA was given s.c. at a dose of 5 mg/kg twice daily for 4 days. 5-HT and 5HIAA were measured by means of reversed phase HPLG-EC as detailed under 'Materials and Methods." Values graphed represent the mean (±S.E.M., n = 3) and are expressed inug/g of Ussue. *Designates significant difference from control group, P < .05, ANOVA followed by Duncan's multiple range test. |
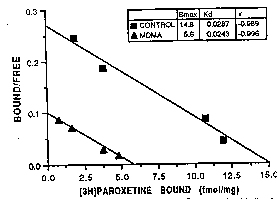 |
Fig. 2. Scatchard analysis of specific in vitro CH]paroxetine binding to membranes prepared from parietal cortex of ON) control and (A) MDMAtreated monkeys. MDMA treatment (5 mglkg twice daily for 4 days) took place 2 weeks previously. Concentrations of rH]paroxetine used ranged from 0.013 to 0.207 nM. Binding studies were performed as described under 'Materials and Methods." Data are from a representative experiment repeated twice with similar results. Kinetic constants (shown in ; t) were calculated with the aid of the computer program EBDA. |
TABLE 1
Mean (±S.E.M) percent reductions in 5-HT, 5-1-11AA and ['H] paroxetine binding 2 weeks after MDMA treatment 5-HT, 5M1AA and [3H]paroxetirbe binding were determined as described under "Materials aM Methods."
| brain region | N | 5-HT | 5-HIAA | [³H]paroxetine |
| FC¹ | 3 | 64.6 ± 5.2 | 56.2 ± 6.1 | 65.5 ± 6.5 |
| HI | 3 | 63.9 ± 4.9 | 72.1 ± 1.9 | 72.3 ± 4.8 |
| CB¹ | 3 | 59.1 ± 7.3 | 68.8 ± 1.7 | 65.3 ± 6.1. |
In the TH, there was still partial recovery of 5-HT (table 2) with complete recovery of 5-HIA.A (table 3). In the HYP, there was near total recovery of both 5-HT and 5-HIAA. by 8 months and, by 18 months, 5-HT and 5-HIAA. levels had risen to 140 and 187% of control values, respectively (fig. 4). ['H] Paroxetine binding studies could not be performed using HYP samples because of insufficient tissue.
Eighteen months after MDMA treatment, the reductions in 5-HT content, ['Hjparoxetine binding and 5-HIAA concentrai did not appear to be as concordant as they had been 2 weeks after MDMA treatment (table 4). For example, in the II, I'Hlparoxetine binding was reduced to a greater extent than 5-HT or 5-111AA. Similarly, in the CH, paroxetine binding was reduced to a greater extent than 5-111AA. (table 4).
Dopaminergic Neurons
Two weeks after drug treatment, concentrations of DA and IWPAC were unaltered in the CH, PR and PFC of MDMAtreated monkeys (table 5). Over time, no changes in the levels of either DA or DOPAC were evident in any of the forebrain regions examined except in the CB2, where DOPAC levels were slightly but significantly reduced (table 5).
Noradrenergic Neurons
Regional brain NE levels were also unaffected 2 weeks after MDMA (table 6). As with DA levels, no changes in regional brain NE levels developed over time in any of the brain regions examined (table 6).
Discussion
The results of the present study indicate that the neurotoxic effects of MDMA in the primate, in contrast to those in the rodent, are longlasting, possibly permanent. Monkeys examined as long as 18 months after MDMA treatment continue to show marked decrements in 5HT levels, I'Hjparoxetine-labeled 5-HT uptake sites and 5-HIAA. concentrations. Because 5-HT, 5-HT uptake and 5-111A.A are the principal presynaptic markers of serotonergic nerve fibers, the present results suggest that serotonergic axons in the primate, unlike those in the rodent, fail to recover from MDMA injury.
Results from two other studies also point to persistent effects of MDMA and related drugs on serotonergic neurons in nonhuman primates. Insel. et al. (1989) have shown that that rhesus monkeys given repeated high doses of MDMA show severe 5HT neurochemical deficits 14 weeks after drug treatment. Similarly, Woolverton et al. (1989) have found that methamphetamine-treated rhesus monkeys show larg~ depletions of brain 5-HT and 5-HIA.A when examined 4 years later. Together with the present results, these findings suggest that the neurotoxic effects of MDMA and related drugs on 5-HT neurons in the primate may be permanent.
As alluded to above, lack of axonal recovery in the MDMAtreated primate contrasts with the vigorous 5-HT axonal recovery that has been observed in MDMA-treated rats (Battaglia et cd., 1988; Molliver et al., 1990) and in rats treated with other serotonergic neurotoxins (Azmitia et al., 1978, Bjorklund et aL, 1981). The basis for this apparent species difference is unclear. It could be related to the fact that the effects of MDMA on 5-HT neurons in the primate are initially more extensive than those in the rodent (Ricaurte et al., 1988a). Alternatively, it could indicate that 5-11T neurons in the primate have less regenerative potential than those in the rodent. Yet another possibility is that the likelihood of axonal recovery is inversely proportional to the distance that an injured axon has to travel before reestablishing synaptic contact. If this hypothesis is correct, lack of recovery in the monkey may be due to the fact that damaged axons in this experimental animal have to travel longer distances than those in the rat before reestablishing synaptic contact (because of the larger brain size of the monkey). Consistent with this proposal is the observation that serotonergic axons in the monkey which have shorter distances to travel (e.g., those innervating the thalamus and hypothalamus) show evidence of recovery, whereas those innervating more distant structures(e.g., cerebral cortex) do not.
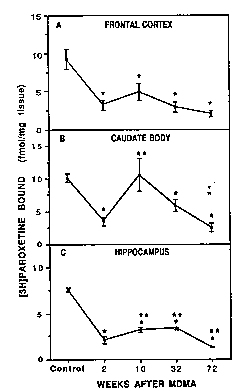 |
Fig. 3. Serotonin uptake sites labeled with rH]paroxetine in various regions of the monkey forebrain 2 weeks, 10 weeks, 8 months and 18 months after MDMA treatment (5 mg/kg twice daily for 4 days). Values were determined from studies in which a saturating concentration (0.207 nM) of [3 H]paroxetine was used. Binding studies were performed as described under 'Materials and Methods.* Data are expressed in pM of [3H]paroxetine bound per mg of tissue. *Designates significantly different from control group, P < .05, ANOVA followed by Duncan's multiple range test. -Designates signilficantly different from 2-week group, P < .05, ANOVA followed by Duncan's multiple range test. |
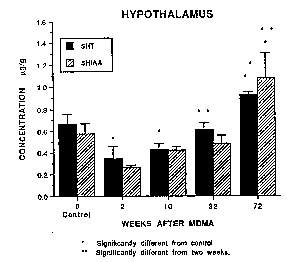 |
Fig. 4. Hypothalamic 5-HT and 5-HIAA concentrations in control monkeys and in monkeys administered MDMA 2 weeks, 10 weeks, 8 months and 18 months previously. Values shown are the mean (±S.E.M., n = 3) and are expressed in ug/g of tissue. *Designates significantly different from control group, P < .05, ANOVA followed by Duncan's multiple range test. -Designates significantly different 2-week group, P < .05, ANOVA followed by Duncan's multiple range test. |
TABLE 4
Mean (±S.E.M.) percent reductions in 5-HT, 5-HIAA and paroxetine binding 18 months after MDMA treatment
Serotonin 5-1-111AA and CHIparoxetine binding were detemined as described under "Materials and Methods."
| brain region | N | 5-HT | 5-HIAA | [³H]paroxetine |
| FC¹ | 3 | 73.7 ± 3.3 | 58.8 ± 9.3 | 77.6 ± 5.3 |
| HI | 3 | 62.5 ± 3.1 | 57.3 ± 3.4 | 82.9 ± 1.0 |
| CB¹ | 3 | 58.8 ± 10.2 | 37.5 ± 7.1 | 74.6 ± 5.9. |
Significantly different from 5-HT and 5-HIAA (P < .05).
Significantly different from 5-HIAA only (P < .05 ).
The early partial, but unsustained, recovery of presynaptic serotonergic markers noted in some brain regions of the MDMA-treated. monkey (HI, CH) deserves comment. First, because a similar partial recovery was observed in an earlier study (Ricaurte et aL, 1988b), it would appear that partial recovery is a consistent phenomenon. Second, the fact that in some brain regions there is parallel recovery of all three presynaptic markers (5-HT, 5-HIA.A and 5-HT uptake sites) suggests that neurochemical recovery is related to axonal regeneration. Consistent with this view are data in the rodent showing that 5-HT neurons have marked regenerative potential Molliver et aL, 1990; Bjorklund et aL, 1981; Mmitia, et aL, 1978). At seeming odds with this interpretation, however, is the protracted time course of recovery, which in some brain regions (e.g., HI) persists for as long as 8 months after MDMA injury. At first glance, such a protracted time course would seem to argue against the notion that neurochemical recovery is related to axonal regeneration. However, it should be recognized that at present, virtually nothing is known regarding 5-HT axonal regeneration in the primate central nervous system. Hence, it would be premature at this point to dismiss the possibility that partial neurochemical recovery reflects an attempt (albeit an unsuccessful one) at axonal regeneration. Of course, the possibility that the observed neurochemical changes do not have a direct morphological correlate, but are purely biochemical (perhaps compensatory) in nature, also needs to be considered.
If an unsuccessful attempt at axonal regeneration underlies the partial recovery of 5-HT markers observed in some brain regions (e.g., CH, HI), the question arises as to whether axons which fail to recover undergo retrograde degenerative changes, and whether such changes proceed back to the level of the nerve cell body. If they do, nerve cell body loss could account for the persistent neurotoxic effects of MDMA in the primate. Studies evaluating this possibility are now in progress, along with morphological investigations aimed at elucidating the significance of increased levels of 5-HT and 5-HUA in the HYP (fig. 4). In particular, the possibility that increased levels of 5-HT and 5HIAA in the HYP reflect serotonergic: hyperinnervation of this brain region is being pursued.
Although findings with presynaptic serotonergic markers point to possible axonal sprouting in some brain regions (e.g., HYP), findings with dopaminergic: and noradrenergic markers do not. For example, DA and DOPAC do not increase over time in either the CH or PR, brain regions which are markedly and persistently depleted of their 5-HT content (table 5). Similarly, NE levels fail to increase over time in any of the brain regions examined (table 6). Thus, there is no indication that either DA or NE neurons undergo collateral sprouting into 5-HT-denervated brain areas. Absence of DA or NE collateral sprouting in the MDMA-treated monkey contrasts with the occurrence of 5-HT sprouting in the 6-hydroxydopaminetreated rat (Snyder et aL, 1986; Blue and Molliver, 1987). This difference could indicate that 5HT neurons have greater sprouting potential than catecholaminergic neurons (though this seems unlikely; see Reis et aL, 1978). Alternatively, age factors could be involved, because serotonergic sprouting in rats occurs only when dopaminergic innervation is removed during the neonatal period (Stachowiak et d, 1994; Snyder et d, 1986).
For the most part, reasonably good concordance was observed in this study among the various presynaptic markers for serotonergic: axons (5-HT, 6-HUA and ['H]paroxetine binding). For instance, 2 weeks after MDMA treatment, all three markers were comparably reduced (table 1). Such concordance, however, was not observed at all time points examined in this study. For example, at 18 months, ['H]paroxetine-labeled 5-HT uptakes sites appeared more reduced than 5-HT and 5-HIAA levels (table 4). Whether the apparent discordance among markers observed at 18 months (and sometimes at other time points) is due to the presence of axons which are not entirely normal is not known, but, as suggested by Battaglia et al. (1988), important to consider. It is also important to consider the possibility that although some discordance between presynaptic markers may reflect a true alteration in axonal structure or function, 3ome of it may be due to experimental variability.
In conclusion, the results of this study indicate that MDMA, a recreational drug of abuse, produces lasting effects on serotonergic neurons in nonhuman primates, with most brain regions showing signs of persistent 5-11T denervation, and others showing signs of reinnervation (TH) and possibly even hyperinnervation (HYP). Why the long-term effects of MDMA in the primate should contrast with those in the rodent is not known, but of interest to determine. Such studies hold promise for enhancing our understanding of neuronal response to injury, and could help identify factors governing axonal regeneration in the central nervous system of primates. Finally, the usefulness of the MI)MA-treated primate for evaluating strategies of neuronal rescue (e.g., neurotrophic factors, transplants) and for elucidating the functional role of 5-11T in the primate brain remains to be explored.
Acknowledgments
We gratefully acknowledge the expert technical assistance of George Hatzidimitriou and Lynda Roggio. This research was supported by a grant from the National Institute on Drug Abuse (DA05707).
References
AZMITIA, E. C., BUCHAN, A. M. AND WILLIAMS, J. H.. Structural and functional restoration by collateral sprouting of hippocampal 5-HT axons. Nature 274: 374-376,1978.
BATTAGLIA, G., YEH, S. Y., WHEARN, K, MOLLWER, M. E., KuWAR, M. J. AND DESouzA, E. B.: 3,4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: Quantification of neurodegeneration by measurement of f'Hlparoxetine-labeled serotonin uptake sites. J. Pharmacol. Exp. Ther. 242. 911-916,1987.
BATTAGLIA, G., YEH AND DE:SouzA, E. B.: MDMA-induced n%urotoxicity. Parameters of degeneration and recovery of brain serotonin neurpns. Pharmacol. Biochem. Behav. 29. 269-274, 1988.
BJORKLUND, A_ W1KLUND, L AND DFscARRIEs, L.: Regeneration and plasticity of central scrotonergic neurons: A review. J. Physiol. 77: 247-257, 1981.
BLUE, M. E. AND MOLLIVER, M. E.: 6-Hydroxydopamine induces serotonergic axon sprouting in cerebral cortex of newborn rat. Brain Res. 32: 255-269, 1987.
BOST, R. 0.: 3,4-Methylenedioxymethamphetemine (MDMA) and other amphetamine derivatives. J. Forens. Sci. 33: 576-587, 1988.
CommiNs, D. L., VOSMER, G., VIRUS, Ii., WOOLVERTON, W., SCHUSTER, C. AND SEIDEN, L.: Biochemical and histological evidence that methylenedioxymethamphetamine (MDMA) is toxic to neurons in the rat brain. J. Pharmacol. Exp. Ther. 241: 338-345,1987.
HABERT, E., GRAHAm, G., TAHRAow, L., CLAUSTRE, Y. AND LANGER, S. Z.: Characterization of ["H] -paroxetine binding in rat cortical membranes. Eur. J. Pharmscol. 118: 107-114, 1985.
INSEL, T. R, BATTAGLIA, G., JOHANSSEN, J.. M~ S. AND DE SouzA, E. B.. 3,4-Methylenedioxymethamphetaniine ("Ec~y*) selectively destroys brain serotonin nerve terminals in rhesus monkeys. J. Pharmacol. Exp. Ther. 249: 713-720,1989.
KLEVEN, M. S., WOOLVERTON, W. L. AND SEIDEN, L. S.: Evidence that both intragastric and subcutaneously administered MDMA produce 5-HT neurotoxicity in rhesus monkeys. Brain Res. 488: 121-125,1989.
KILPATRICK, I., JONES, M. AND PRILLIPSON, 0.: A serniautomated analysis method for catecholamines, indoleamines, and some prominent metabolites in microdissected regions of the nervous system: An isocratic HPLC technique employing coulometric detection and minimal sample preparation. J. Neurochem. 46(6): 1865~1876, 1986.
KOTAKE, C., HEFFNER, T., VOSMER, G. AND SEIDEN, L.: Determination of doparnine, norepinephrine, serotonin and their major metabolic products in rat brain by reverse-phase ion pair high performance liquid chromatography with electrochemical detection, Pharmacol. Biochem. Behav. 22: 85-90,1985.
MOLLIVER, M. E., MAMOUNAs, L. A. AND CARR, P.: ReinnCryation of cerebral cortex by 5-HT axons after denervation by psychotropic amphetamine derivatives. Soc. Neuroaci. Abstr. 15: 417, 1989.
MOLLIVER, M. E., BERGER, U. V., MAMOUNAS, L. A., MOLLIVER, D. C., 0'HEARN, E. G. AND WILSON, M. A.: Neurotoxicity of MDMA and related compounds: Anatomic studies. N.Y. Acad. Sci. 600: 640-664, 1990.
O'lIzARN, E. G., BATTAGLIA, G., DE SOUZA, E. B., KUHAR, M. J. AND MOLWYER, M. & Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause ablation of serotonergic axon terminals in forebrain: Immunocytochemical evidence. J. Neurosci. 8: 2788-2803, 1988.
Rgis, D. J., Ross, R. A., GiLAD, G. AND JOH, T. H.: Reaction of central catecholaminergic neurons to injury: Model systems for studying the neurobiology of central regeneration and sprouting. In Neuronal Plasticity, ed. by C. Cotman, pp. 197-226, Raven Press, New York, 1978.
RICAuRTE, G. A., FORNO, L. S., WILSON, M. A., DELANNEY, L. E., IRWIN, L, MOLLIVER, M. E. AND LANGSTON, J. W.: MDMA selectively damages central serotonergic neurons in the primate. J. Am. Med. A~. 260: 51-55, 1988a.
RiCAuRTE, G. A., FORNO, L. S., WIENER, S. G., DELANNEY, L. E.. IRWIN, 1. AND LANGSTON, J. W.: Effects of MDMA on central serotonergic neurons in nonhuman primates: Permanentor transient? Soc. Neurosci. Abstr. 14:558,1988b.
RICAuRTE, G. A.: Studies of MDMA-induced neurotoxicity in nonbuman primates: A basis for evaluating long-term effects in humans. In Pharmacology and Toxicology ofAmphetamines and Related Designer Drugs, ed. by K. Asghar and E. DeSouza, pp. 306~322 NIDA Research Monogr. 94 p. 306-322,1989.
SCHMIDT, C. J.: Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J. Pharmacol. Exp. Ther. 240: 1-7,1987.
SNYDM A. M., ZIGMOND, M. J. AND LUND, R. D... Sprouting of serotonergic afierents into striatum after dopamine-depleting lesions in infant rats: a retrograde transport and immunocytochemical study. J. Comp. Neurol. 245: 274-281,1986.
STACHOWIAK, M. K, BRUNO, J. P., SNYDER, A. M., STRICKER, E. M. AND ZIGMOND, M. J.: Apparent sprouting Of Btriatel serotonergic terminals after doparnine-depleting lesions in neonatal rats. Brain Res. 29 1: 164-167, 1984.
STONE, D. M., STAHL, D. S., HANSON, G. L. AND GmB, J. W.: The effects of 3,4methylenedioxymethamphetamine (MDMA) and 3,4methylenedioxyamphetamine (MDA) on monoarninergic systems in the rat brain. Eur. J. Pharmacol. 128:41-48,1986.
WILSON, M. A.. RICAURTE, G. A. AND MOLLIVER, M. E.: Distinct morphologic cl~ of serotonergic axone in primates exhibit differential vulnerability to the psychotropic drug 3,4-methylenedioxymethamphetamine. Neuroscience 28:121-137,1989.
WILSON, M. A. AND MOLLIVER, M. & Regeneration in the CNS following MDAinduced neurotoxicity: Pharmacologic evidence for selective reinnervation by fine serotonergic axons. Soc. Neuroaci. Abstr. 16: 1032, 1990.
WOOLVERTON, W. L., RiCAurm, G. A., FORNO, L. S. AND SEIDEN, L. S.: Longterm effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 486: 73-78,1989.
Send reprint requests to: George A- Ricaurte, M.D, Ph.D., Assistant Professor of Neurology, Francis Scott Key Medical Center, Johns Hopkins School of Medicine, 4940 Eastern Avenue, Baltimore, MD 21224.
Last Updated (Monday, 20 December 2010 19:37)












