RECENT DEVELOPMENTS IN CANNABIS CHEMISTRY
| Articles - Cannabis, marijuana & hashisch |
Drug Abuse
Alexander T. Shulgin, Ph.D. (a)
(a) Research Fellow Langley Porter N.P.I. San Francisco, California
INTRODUCTION:
The marijuana plant Cannabis sativa contains a bewildering array or organic chemicals. As is true with other botanic species, there are representatives of almost all chemical classes present, including mono-and sesquiterpenes, carbohydrates, aromatics, and a variety of nitrogenous compounds. Interest in the study of this plant has centered primarily on the resinous fraction, as it is this material that is invested with the pharmacological activity that is peculiar to the plant. This resin is secreted by the female plant as a protective agent during seed ripening, although it can be found as a microscopic exudate through the aerial portions of plants of either sex. The pure resin, hashish or charas, is the most potent fraction of the plant, and has served as the source material for most of the chemical studies.
The family of chemicals that has been isolated from this source has been referred to as the cannabinoid group. It is unique amongst psychotropic materials from plants in that there are no alkaloids present. The fraction is totally nitrogen-free. Rather, the set of compounds can be considered as analogs of the parent compound cannabinol (I), a fusion product of a terpene and a substituted resorcinol. Beyond the scope of this present review
are such questions as the distribution of these compounds within the plant, the botanic variability resulting from geographic distribution, the diversity of pharmacological action assignable to the several distinct compounds present, and the var ious preparations and customs of administration. This presentation will be limited primarily to chemical structure and synthesis, with only passing comments on the topics of biosynthesis and human use.
EARLY STRUCTURAL STUDIES:
A brief review of the early analytical and synthetic studies in the area of cannabis chemistry is necessary as a background for the discussion of recent developments.
Cannabinol, I, was the first compound isolated from the resin of Cannabis sativa as a
pure chemical substance. Its synthesis established the carbon skeleton that is common to the entire group of cannabinoids. This 21-carbon system can be best described as an amalgamation of a 10-carbon monoterpene and 5-amylresorcinor (olivetol). The terpene half is shown to the left of the dotted line above. In cannabinol, it is completely aromatic and represents a molecule of cymene. In all of the remaining cannabinoids isolated from the native resin it is found in a partially hydrogenated state, usually with a single double bond remaining. The resorcinol moiety, olivetol, is the portion that is represented to the right of the dotted line. It is to be found as an invariable component of all the constituents of the resin, although as will be described below, it often appears as the corresponding carboxylic acid, olivetolic acid. Synthetic variations on the amyl group have proved to be one of the most rewarding series of studies in this area of chemistry, and represents most of the structure-activity investigations that have been pursued. This early synthetic work will be described only briefly, for it has involved analogs of tetrahydrocannabinol which display the terpene double bond in an unnatural position. The most recent chemical advances represent syntheses of the exact natural products, and these will be the body of this discussion.
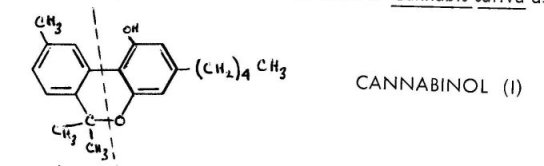
A brief comment is desirable concerning the various modes of ring numbering that have been employed in this area. Four separate and distinct methods have been employed in the chemical literature. Each has a virtue over the others, but each carries with it limitations. These complications arise from the fact that, in many of the cannabinoids present in the natural resin, the oxygen-containing ring (the pyran ring in cannabinol above) is not present. These materials as isolated are dihydroxybiphenyls, with no heterocyclic ring.

The first of these numbering systems was introduced by Todd in England. It considers these substances best referred to as variously substituted pyrans . Thus, the base numbers are assigned to the pyran ring, and the 4, 5-bond between the two remaining rings defines the 1-position of each. The first of these, the "prime" set is applied to the terpene ring and proceeds clockwise. The second, or "double prime" set, refers to the aromatic ring and proceeds counterclockwise. Thus the carbon atoms common to the pyran ring are numbered one and two in each case.
A related numbering system is one that employs the Chemical Abstracts convention. Here these substances are considered as substituted dibenzopyrans, and numbered starting with the first unfused position of the aromatic ring. The obvious disadvantage of both of these systems is that the numbering must be totally changed in those isomers in which the central pyran ring is open.
To compensate for this latter limitation, a biphenyl numbering system has come into usage, primarily in Europe.

Here, the various substitution positions are sequentially numbered from the central carbon bond of biphenyl. The terpene ring is fundamental, and the aromatic ring commands the prime numbers. The advantages of this system are reciprocal to those mentioned above. The open ring compounds are easily numbered, but there is no general convention that extends to modifications that include the pyran ring. Note should be made of the fact that the course of numbers in the terpene ring is opposite to that of the other systems.
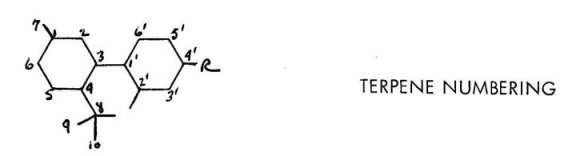
Most broadly used today is a numbering system that recognize both the terpene nature and the aromatic nature of the two different parts of the molecule. Thus the terpene is numbered in a manner that is conventional for it, i.e., from the ring carbon that carries the branched methyl group. This is in turn numbered seven, and the remaining three carbons of the isopropyl group are then numbered sequentially. The aromatic ring assignments are straight forward. The over-whelming advantage here is that this numbering system is applicable whether the center ring is open or closed, and further it can be extended to new compounds that may be isolated as long as they can be represented as a combination of a terpene and an aromatic ring. The only exception is in the instance that the terpene portion is an open chain. Examples of this are known, and their numbering system will be mentioned later.
A brief discussion of the early synthetic efforts in this area is informative, as it provides the only systematic correlation between chemical structure and biological activity. Early in these chemical studies, at about the time of World War II, two compounds were isolated from the red oil fraction of cannabis. One was an optically active tetrahydrocannabinol whidi carried a double bond in the terpene ring. The other was the open-ring counterpart; it contained two phenolic groups and two double bonds. It was also optically active, and was named cannabidiol.

The location of the exocyclic double bond in the latter compound was readily established, both by its easy conversion into tetrahydrocannabinol (THC), and by the generation of formaldehyde on ozonolysis. The endocyclic double bond proved to be extremely difficult to locate. It was known not to be in conjugation either with the exocyclic counterpart or with the aromatic ring. This still left three possibilities,
Although this problem has only recently been solved, during the period of these initial isolations and characterizations, synthetic explorations were numerous. As two generally different synthetic methods were employed, and two different biological assays as well, it is quite difficult to interrelate these studies. The first of the tetrahydrocannabinol syntheses was that of Todd and co-workers in England. Their scheme consisted in the fusion of a terpene such as pulegone with olivetol, thus producing the three ring product.
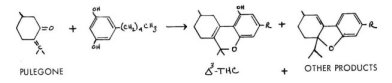
Pharmacological evaluations were made employing rabbit corneal areflexia; unfortunately there are no available correlations between this response and human intoxicative potency. Two serious complications have appeared in this approach. The pulegone employed has been shown to be of uncertain optical purity, thus leading to optically active products of inconsistent composition . Further, the actual nature of the condensation leads to structural isomers. This was due in part to the contamination of pulegone as isolated from natural sources with isopulegone, and in part to a sensitivity to the specific nature of the condensation agent.
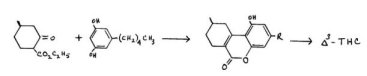
A more satisfactory scheme was developed by Adams and his co-workers at Illinois. In this process a completely synthetic keto-ester was condensed with olivetol, and the resulting lactone converted in a separate step to the grdimethyl product. Again, as with the pulegone synthesis above, the unnatural P. isomer of THC was the principle product. This, and related honnologs, were titrated pharmacologically by dog ataxia assay. They were found to be qualitatively similar although quantitatively less active than natural THC isolated from the red oil. This unnatural but reproducably available isomer was taken as a reference standard for an extensive study of structural modifications.
A complete review of these studies would be out of place in a presentation designed to emphasize recent developments. Comment should be made, however, on the importance of the amyl group on the aromatic ring. It was found that, in the straight chain series, a maximum activity was observed at the 6-carbon (hexyl) substitution. This material was about twice as active as the reference amyl compound in the ataxia analysis, and has undergone extensive clinical study under the name of Synhexyl or Pyrahexyl. The replacement of a straight chain with one branched at the alpha- carbon, led to the alpha-methylhexyl counterpart, with the increase of potency of a full order of magnitude. The studies that have resulted in the development of alpha, beta- dimethyl analogs, have led to the dimethylheptyl-analog, a compound known as DMHP or Adams' nine-carbon compound. It is yet a full order of magnitude more potent than the methylhexyl material mentioned above, i .e. 500x more potent than the reference 63-THC.
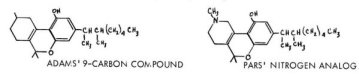
Its activity has been confirmed in human subjects, and just recently the tedious task of its separation into the eight possible isomers has been reported by Aaron. Quite recently a Nitrogen analog of this compound has been prepared by Pars, and appears also to be biologically active.
RECENT CHEMICAL ANALYSES:
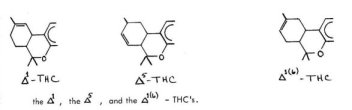
In recent years, immense strides have been made in the area of the chemistry of the cannabinoids of C. sativa. The development of sophisticated spectroscopic instruments, particularly in nuclear magnetic resonance, has settled the question of the exact isomeric configuration of the elusive double bond in the terpene ring, and has established the stereoconfiguration about the 3,4-position. The principle isomer present in the red oil is A 1-tetrahydrocannabinol_11 , in which the 3,4-hydrogens are orieted trans- to one another. The open-ring counterpart of Al -THC is consequently A -3,4- trans-cannabidiol (Ill). It has been reported that Al -(6)-- isomer of THC is also present in the native resin, but this is uncertain as it could have arisen as an artifact of isolation.
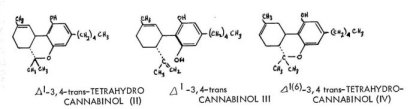
None of these fine structural assig9ments could have been possible, however, without the development of elegant methods of fractionation and isomer separation concurrently with the instrument techniques. The procedures of column and thin layer chromatography have made possible not only the isolation of characterizable amounts of isomerically pure materials, but have led to the discovery of a host of additional chemicals that had heretofore been unknown.
An acidic fraction has long been known to be present in the red oil of C. sativa. Some ten years ago an acid was isolated which proved to be, after structural correction for the now-known location of the endocyclic double bond of Al-THC, the benzoic acid that corresponds to Ill, i.e., cannabidiolic acid, V. This material corresponds to canna-bidol both in the double bond location and in the trans- configuration about the 3,4-bond. The presence of this and other acidic materials in the resins isolated from marijuana grown in the colder northern latitudes suggests their roles as biosynthetic precursors to the more neutral aromatic, active, fractions.
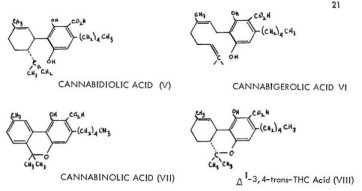
Careful chromatographic separation of this acidic fraction into individual components has afforded three more aromatic carboxylic acids. These are cannabigerolic acid (VI) that upon decarboxylation could yield cannabioerol (v.i.), cannabinolic acid (VII) which can give rise to cannabinol (I), and A' -3,4-trans-tetrahydrocannabinolic acid (VIII) which can be converted to, and which may well be argued as being a normal biosynthetic presursor to, &Al -THC. The possible roles of these acids as biological intermediates which could lead to the neutral (phenolic) cannabinoids, will be discussed below.
In the chromatographic analysis of the less plentiful components of the resinous fraction of C. sativa several additional phenolic components have been isolated and assigned tentative chemical structures.
A one-ring resorcinol has been separated that, upon spectroscopic analysis, appeared to be the simple fusion of an open-chain terpene and olivetol. This material cannabigerol (IX) has had its structure proven by synthesis. The fusion of this terpene with olivetolic acid would then give rise to the above-mentioned acid, cannabigerolic acid (VI). This type of open-ring terpene compound presents yet another numbering system, being determined by the eight-carbon chain attached to the olivetol nucleus. The numbering by convention starts at the distal end of the chain, as shown in IX and X.
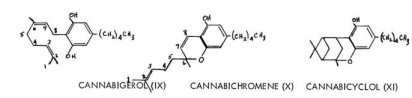
Two additional phenolic components have recently been described. Cannabichromene, X, is an open-chain benzopyran containing a carbon system closely related to the material cannabigerol but with heterocyclic ring closure. A phenol named cannabicyclol (XI) has also been isolated which, lacking any unsaturation whatsoever, has been assigned the structure shown. These two hypothetical structures lack support from synthetic studies. These fusions of terpenes and aromatics have suggested several of the recent synthetic approaches into this area, and also provide the basis of biosynthetic paths, to be discussed.
RECENT CHEMICAL SYNTHESES:
An expected correlary to the establishment of the tools of structural analysis of C. sativa, was their use in the development and evaluation of synthetic techniques. It must be noted that, in this same period of time -- the last four years or so --, no less than six separate and mutually confirmatory syntheses within this family of compounds have appeared in the chemical literature. Three of these represent modifications of the olivetol ring providing the basis for the construction of the terpenacious half of the cannabinoid molecule. The remaining three syntheses employ the reactivity of the resorcinol system itself and, in effect, bring a terpene into reaction with it.
The first of these procedures can be illustrated by the general reaction between citral and the lithio-derivative of the dimethyl ether of olivetol . The first description of this reaction was advanced by Mechoulam and Gaoni. The coupling product shown undergoes an internal rearrangement to yield the trans- isomer of the dimethyl ether of cannabidiol Demethylatipq leads to cannabidiol itself, and cyclization provides a mixture of the 11' and the 111 k6) -isomers of THC. The over-all yield in this procedure is small.
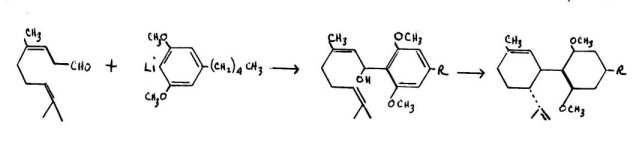
Taylor, Lenard and Shvo have confirmed this reaction scheme and have found that the Al (6) -trans isomer of THC to be a principle product. They did obtain after chromatographic separation, a reasonable yield of the L11-THC isomer. Very recent modifications of the procedure of Taylor have been investigated by Gaoni and Mechoulam, in which they use BF3 rather than HC1 as the condensation agent between the terpene and olivetol. They have observed a 20% yield of the stereo-specifically proper isomer of the L11-THC. The details of these most recent studies have not yet been published.
Two more syntheses have been described which employ modifications of the olivetol molecule. Both effect the construction of the terpene ring through some form of Die Is-Alder reaction, but the necessary intermediates are derived in different ways.
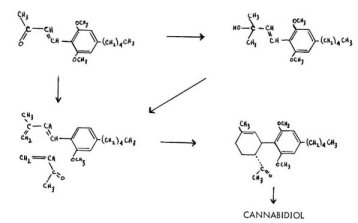
In one of these processes, the diene is attached to the olivetol nucleus, between the two oxygen functions. This diene has been obtained through two separate procedures. In one, Korte, Dlugosch, and Claussen converted the appropriate ketonic intermediate directly to the diene through a Wittig reaction. In the other, Kochi and Matsui dehydrated the carbinol that resulted from a Grignard reaction on this ketone. In both cases, they achieved an identical diene that cyclized readily with methyl vinyl ketone. This reaction led to a stereospecifically correct bicyclic methyl ketone which, upon a Wittig methylene replacement, led to the dimethyl ether of cannabidiol. The yields are poor, but both the stereo-configuration and the double-bond location are correct for the natural orientation.
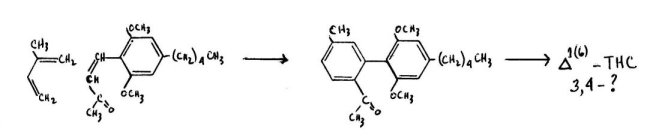
The other Die Is-Alder synthesis involves a scheme in which the dieneophyle is itself attached to the olivetol nucleus. Here either the cinnamic acid or the corresponding methyl ketone is employed as a condensing agent with isoprene. Korte, Hackel and Sieper have reported this reaction with the styryl methyl ketone and have found that the isoprene orientation is proper. After the necessary Wittig reaction, they found that the resulting (6)-cannabidiol did not have an assignable configuration about the 3,4-bond. A modification of this approach has been described by Jen, Hughes and Smith in which, through the employment of the free cinnamic acid itself, a product is obtained that is not only properly oriented with regard to the isoprene molecule, but is also appropriately trans- about the eventual 3,4-terpene bond. This carboxylic acid has been resolved into its optical isomers. These separate enantiomorphs have been appropriately methylated, cyclized, and finally demethylated to provide both the natural and the unnatural optical isomers of P1 (6)-THC. In neither of these reactions is the yield good, and it is only in the second example that the appropriate stereoconfiguration is obtained. This must be isomerized at an additional expense in yield, to the natural 1-isomer.
The three remaining synthetic procedures all employ olivetol as a free resorcinol, not protected or activated in any manner. The first of these examples represents a complete construction of the terpene ring through synthetic procedures, but the last two involve reactions with natural or near-natural terpenes, and so might cast light on those biosynthetic pathways actually effected in the plant in the in vivo production of these cannabinoids.
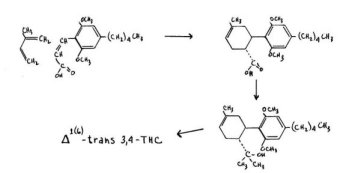
Fahrenholtz, Lurie and Kierstead have reported the syntheses of both the 1- and the 1 (6)_3,4-trans-tetrahydrocannabinol through a synthetic process that represents a complete construction of the terpene ring. The condensation of olivetol with acetoglutarate yields a cyclic product, a benzopyran. This lactone is converted to a three-ring system which carries a ketonic group at the one-location of the terpene ring.
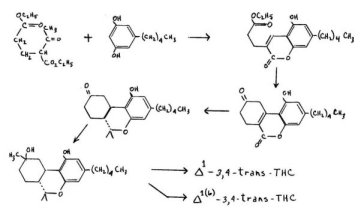
This lactone carries a 3,4-double bond, but it can be converted through appropriate protection and methylation, to the 2, 3-conjugated counterpart with a geminal methylation. The resulting (trans) cyclohexanone is easily methylated and dehydrated to a mixture of THC's which can be separated chromatographically. The stereoconfiguration is correct but the over-all yields are poor.
Mechoulam, Braun and Gaoni have reported a total synthesis that starts from the readily available terpene, pinene. This is oxidized to tke allyl alcohol, verbinol, and then condensed with olivetol to produce a heroic mixture of products. Olivetyl pinene can be chromatographically separated and converted into the 61(6)-THC product, which can in turn be isomerized and so converted into the natural /11-counterpart. In this reaction both the stereo-specificity and the absolute optical configuration can be controlled, but the necessary separations again limit the procedure to the preparation of only small amounts of end-products.
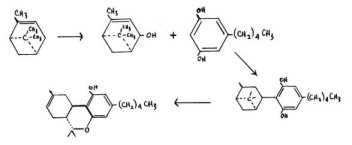
The most recent synthesis in this area has been reported by Petrzilka, Haefliger, Sikemeier, Ohloff and Eschenmoser. They have des&ibed the reaction between unsubstituted olivetol and the easily synthesized terpenol (+)-trans-p-menthadien-2,8-ol. This leads directly to the stereospecifically correct isomer of cannabidiol. The yields are quite good (ca. 25%) and the general process suggests cn easy entry to the study of structural isomers that carry the naturally correct optical and stereo-configurations.
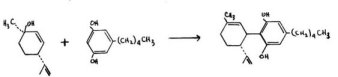
BIOSYNTHETIC CONSIDERATIONS:
From the onset of this review it has been emphasized that the entire family of the cannabinoids can be considered as a combination of the structures of a terpene and a resorcinol. In fact, many of the recently evolved syntheses within this family have employed just such a combination, with chemical modifications as might be dictated by the reaction conditions. It has been mentioned that the several carboxylic acid components of the resin may serve as precursors to this family. This is supported by the observation that in the colder climates, in the Northern regions which provide shorter growing periods and generally cooler conditions, the marijuana that is harvested is known to be more raw, to contain a greater percentage of acidic components, and to be less biologically active.
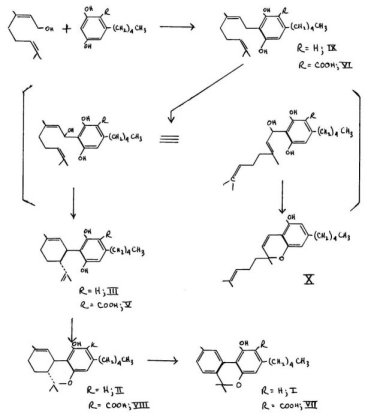
Mechoulam and Gaoni have presented an argument that not only olivetol but olivetolic acid may serve as the condensing moiety for the terpene component. It can be argued that geranol, upon appropriate activation, could condense with olivetol or with olivetolic acid to yield cannabigerol. This reaction has been achieved in vitro. This compound can then undergo an alpha-oxidation to yield cannabidiol through hydroxy elimination, or cannabichromene through addition. The remaining components of the cannabis resin are then explainable by appropriate steps of cyclization, dehydrogenation, and decarboxylation. These transformations are outlined in the flow diagram in which chemicals known to be present are numbered in accordance with their presentation above. The one material of established structure missing from this scheme is the Zi 1 (6) _ 3,4-trans-THC, IV. lt can be readily prepared in the laboratory by the acid-catalysed isomerization of the4 I - counterpart, and such a conversion could certainly occur in the intact plant.
PSYCHOPHARMACOLOGICAL CONSIDERATIONS:
The psychological aspects of the intoxication attributable to marijuana have been widely noted but poorly defined. The psychotropic consequences of an effective dosage have been described as being euphoristic, intoxicating, elevating, depressing , and nauseating. Although often conveniently pigeonholed under the contradictory terms "sedative hypnotic", upsYchotomimetic" or "deliriant", the truth is that there is no reliable way to anticipate just what pharmacological reaction will be experienced by a given individual upon a given occasion. The broad spectrum of effects ranging from elation to sedation may be in part attributed to the variable composition of chemicals present in the plant, and to the fact that their individual pharmacological properties are to a large extent not known.
Human studies have been conducted by Isbell on isolated /11-THC, and by Gaoni and Mechou lam on Pi (6) -THC; both have been shown to be effective psycho - tomimetics. Korte has established that cannabichromene is inactive in humans. Other than these three comments, however, there is no known human pharmacology on the individual components in marijuana. Animal studies of several of these chemicals have been conducted at length, and these show, in addition to the ataxia and areflexia comments made earlier, indications of sedative properties and an antibacterial action.
Some comments concerning the future of clinical studies concerning these pure components of cannabis are in order. It must be apparent that the natural resin is still
the best source for any of the components that have been described. The studies mentioned, by Isbell, were on material isolated from the plant. The ,Mechoulam experiments were cons-ducted with synthetic materials, but they were available only in milligram amounts. Only two of the synthetic procedures that have been discussed could reasonably be extended to allow the production of the quantities of material required for proper clinical evaluation,
but even here the amounts that could be produced are modest. The cost of large-scale production would discourage most investigators. Thus for the time being, it must be assumed thatespecial studies on individual components will be conducted on isolates from natural sources.
There has recently been much discussion of a "synthetic' •THC" that is widely available in our drug subculture. lt is certainly possible that this material may be an extract from plant sources, or a synthetic material similar to THC but employing a route of preparation yet unreported in the scientific literature. Not impossible would be the preparation of a jumble of products by a process such as the fusion of pulegone and olivetol. The resulting resin may well contain some active component. However, in light of the inherent expense involved in the synthesis of any single active material, it seems far more reasonable to assume that this "synthetic THC" is a material dissimilar to any cannabis component and that merely presents a pharmacological effect acceptable as a substitute. The samples that have come to my attention have been salts of nitrogen-containing compounds similar to, but not identical with, benactyzine.
The entitling of this material as a cannabis derivative has, however, surely played some role in the recent, somewhat hasty, action on the part of the federal government. The Bureau of Narcotics and Dangerous Drugs has issued a prescription specifically against synthetic materials, by the addition of the names of Al, Al (6), and A3-THC's to the list of synthetic substances officially considered as dangerous drugs. lt is most un-
fortunate that these materials, which can be obtained by legitimate scientific investigators only with the greatest difficulty, should now become virtually unobtainable, whereas
their availability on the street, heretofore negligable, shall become no less so.
There is obviously a wealth of pharmacological potential latently available in Cannabis sativa, and now that many of the component factors are potentially available as pure discreet compounds it is hoped that these values will be forthcoming. Many of these materials are certainly active biologically, and there is a long reputation for safety. lt is axiomatic that some of them will fill a medical need.












