Chapter 3 The pharmacology of cannabis: issues for understanding its use
| Manuals - Cannabis Reader |
Drug Abuse
Keywords: cannabinoids — cannabis — pharmacology — physical effects — THC
Setting the context
To understand cannabis, it is helpful to have a knowledge of the pharmacology of the drug. What are the psychoactive effects of the substance, and what physical and neurological changes are brought about by the product? What can be said about the
varying effects of dosage, route of administration, the type of product (herb, resin, oil), and the use environment?
Scientific knowledge about the pharmacology of cannabis has seen substantial progress in the last three decades. In addition to substantial work in neuroscience, cannabinoid research accelerated following the discoveries in the late 1980s of cannabinoid-like chemicals produced by the body, known as 'endocannabinoids'.
As with much science, much of the literature on cannabis is technically challenging, especially for those approaching drugs from disciplines such as sociology and political science. In addition, there is a glut of information in the scientific journals: a Medline search on 'cannabis pharmacology' reveals over 3 500 articles, and many more are published each month. Meanwhile, users seeking to explore the science of cannabis are likely, sooner or later, to encounter disinformation and inaccuracy. User reports are by nature subjective, and growshop information is compromised by the incentive to sell. Pro-cannabis lobbying information is skewed towards innocuous and euphoric effects or favourable comparisons with alcohol. Prohibitionist literature emphasises the risks
of cannabis smoking without placing sufficient emphasis on the sought-after effects of cannabis.
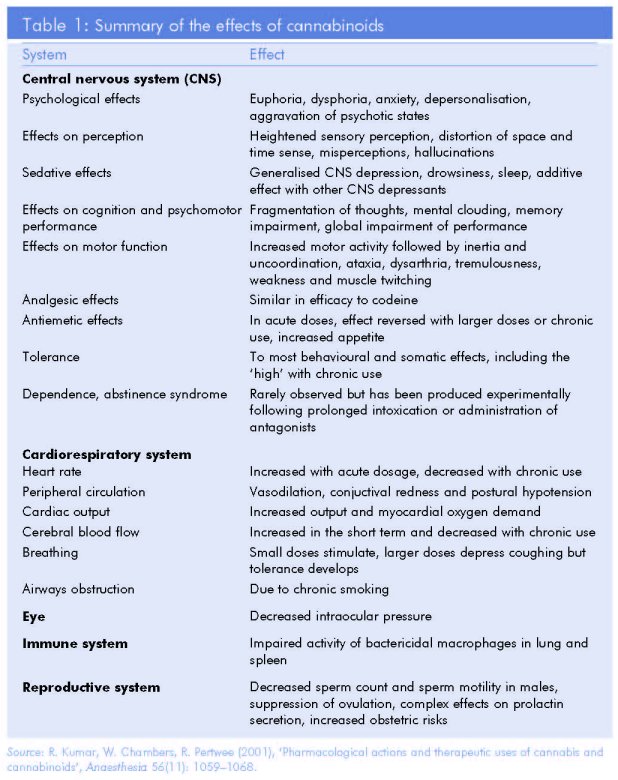
Fortunately, help is at hand for those first approaching the subject. A number of initiatives have sought to provide information that is simultaneously accurate and accessible, and valuable publications exist for a variety of audiences (see Further reading, below). One of the products of a more didactic approach is the simplified summary above by Kumar et al., republished in a number of government monographs since its first appearance in 2001 (Table 1). The chapter that follows, by a leading authority based at Trinity College Dublin, provides a short summary of what is known to date about the pharmacology of cannabis. A glossary is provided to assist nonspecialists.
Further reading
Handbooks for research scientists
Di Marzo, V. (ed.) (2004), Cannabinoids, Kluwer Academic/Plenum Publishers, New York.
Pertwee, R. (ed.) (2005), Cannabinoids (Handbook of experimental pharmacology), Springer, Heidelberg.
Broader scientific research on cannabis and cannabinoids
Grotenhermen, F., Russo, E. (eds) (2002), Cannabis and cannabinoids: pharmacology, toxicology, and therapeutic potential, Haworth Press, Binghamton.
Iversen, L. (2000), The science of marijuana, Oxford University Press, Oxford.
Joy, J. et al. (1999), Marijuana and medicine: assessing the science base, National Academy Press, Washington DC.
Kalant, H., Corrigal, W., Hall, W., Smart, R. (1999), The health effects of cannabis, Centre for Addiction and Mental Health, Toronto.
Raynaud, M. (ed.) (2004), Cannabis et Santé, Flammarion Médecine-Sciences, Paris.
Sociedad espariola de investigaci6n sobre cannabinoides (2002), Gula B6sica sobre los Cannabinoides, SEID, Madrid.
Reviews for practitioners and clinicians
Ashton, H. (2001), 'Pharmacology and effects of cannabis: a brief review', The British Journal of Psychiatry 178: 101-106.
Fact files for users
EMCDDA (2007), Drugs profiles: cannabis, European Monitoring Centre for Drugs and Drugs Addiction, Lisbon
www.emcdda.europa.eu/index.cfm?nnodeid =25484
Karila, M., Reynaud, L. (2006), Fiche Technique, Janvier 2006: Le Cannabis, Comité d'éducation sanitaire et sociale de la pharmacie française, Paris.
MIND (2006), Fact sheets: the psychological effects of cannabis, MIND, London.
Summaries of national government reports
Ben Amar, M. (ed.) (2001), Cannabis, drogues, santé et société 2 (2), Montreal. Rodin Foundation (2005), Le cannabis: document de travail, Brussels www.rodin-foundation.org/PDF//publications/43fr.pdf
See also the grey literature list in the Appendix to Volume 1 of this monograph (p.300).
The pharmacology of cannabis: issues for understanding its use
Desmond Corrigan
Abstract
The drug products obtained from the plant Cannabis sativa contain many different chemicals. The most active are the phytocannabinoids, such as THC, which exert their psychoactive effects by binding to specific receptors within the brain and other parts of the body. The existence of a complex endocannabinoid system within humans and other animals and the interaction between the phytocannabinoids and this system explains many of the rewarding, dependence-producing effects of cannabis drugs as well as their influence on movement, coordination, reactions, memory and learning, especially since the brain regions implicated in these effects are richest in cannabinoid receptors. Cannabinoids are highly fat-soluble and their metabolism and slow excretion from the body distinguishes them from other drugs, such as alcohol. The slow elimination of THC explains the low intensity of withdrawal symptoms and also why urine tests following consumption test positive for cannabinoids for much longer than for most other psychoactive drugs (up to two weeks).
Cannabis drugs
The plant Cannabis sativa L. is the source of a number of drug products. While herbal cannabis (or marijuana) consists of dried plant parts, the main ingredient in cannabis resin (or hashish) is the resin secreted by the glandular hairs found all over the plant but mainly around the flowers. In addition to these two kinds of preparation, which have been used since time immemorial, hashish oil is extracted by use of a solvent (e.g. acetone) and evaporated. In addition, the cannabis plant can be used as a source of hemp fibres, as well as hemp seeds and fatty oil.
The flowering tops and leaves of the plant Cannabis sativa secrete a resin containing about 60 terpenophenolic compounds which are called cannabinoids, to distinguish the plant compounds from the endogenously occurring endocannabinoids found in most animals, especially humans. The highest amount of cannabinoids has been found in the flowering tops, followed by the leaves, whereas only small amounts are found in the stem and roots. While for many years herbal cannabis typically showed a lower cannabinoid content than preparations (resin and oil), innovation in cultivation techniques, pruning and seed selection have enabled marijuana growers to match or exceed the potency of resin (see King, this monograph).
Phytocannabinoids
The main cannabinoid is A9—tetrahydrocannabinol (THC), which is recognised as the major psychoactive euphoriant responsible for the characteristic intoxication
('high') which follows the smoking or ingestion of cannabis. High THC doses produce hallucinogenic effects. In addition to THC, several less potent metabolites and related compounds, such as the also psychoactive A8-THC and cannabinol are found in the cannabis plant. Another major compound is cannabidiol (CBD), which has antagonistic effects to THC because it is a sedative compound. The ratio of THC to CBD in the plant is significant in terms of psychoactivity and is genetically determined.
A number of chemotypes exist within cannabis. These are plants which are visually and botanically identical but which are chemically dissimilar. One type referred to as the fibre- or hemp-type contains predominantly CBD and only trace amounts of THC (less than 0.3% THC according to Commission Regulation (EC no. 327/2002)).
Conversely, drug-type plants produce predominantly THC with trace quantities of CBD. The issue is further complicated by the existence of an intermediate plant which contains approximately equal amounts of both THC and CBD. The concentrations of these and other cannabinoids vary enormously in practice depending on plant breeding and cultivation techniques and on post-harvest handling. The question of the potency of cannabis drugs, usually expressed in terms of THC content, is dealt with in the chapter by King (this monograph, p.239). THC is a highly unstable compound, breaking
down in air and light to a number of inactive molecules, one of which, cannabinol (CBN), is commonly found in cannabis products as they age. Other relatively abundant cannabinoids include cannabigerol (CBG) and cannabichromene (CBC) but in general little is known about the biological activities of these and the remaining less frequently occurring molecules.
Most pharmacological research has focused on THC and CBD. However, while THC is responsible for many of the effects of cannabis drugs, it is important to bear in mind that THC and cannabis are not synonymous for a number of reasons.
Firstly, THC does not exist as such in the plant material but rather it is found as an acid (THCA), as is CBD. These acids (HCA and CBDA) decompose slowly during storage to the corresponding chemically neutral but pharmacologically potent THC and CBD. This conversion is speeded up by the high temperatures involved in smoking and to a lesser extent by cooking or baking the drugs. Secondly, the THC/CBD ratio can markedly
alter the effects of the drugs. Thirdly, some of the non-cannabinoid compounds from the plant may modulate the pharmacological effects of the cannabinoids. Terpenoids, which are responsible for the characteristic smell of cannabis, have been postulated as influencing the effects yet experimental evidence is scarce. Some 1% by weight of the plant is composed of a mixture of 20 flavonoid compounds which are well known as antioxidants and which also scavenge damaging free radicals. Whether the quantities which survive the pyrolysis reactions involved in smoking cannabis are sufficient for activity is unknown (Musty, 2004).
Pharmacokinetics and metabolism
The dose of THC needed to produce effects in humans ranges from 2 to 22 mg (Adams and Martin, 1996). It is estimated that only 1% of the THC content of a 'joint' is found in the brain after smoking; hence, only 2-4411g of THC enters the brain in humans. Given the significant variation in cannabinoid content in the crude drugs and also in the weights of those crude drugs incorporated into 'joints' (Buchanan and O'Connell, 1998), there is little comparability or standardisation of dosages of THC and the other cannabinoids in practice.
THC is rapidly absorbed after inhalation of cannabis smoke and it is detectable in plasma within seconds. Between 10 and 50% of the THC in the drug reaches the bloodstream. Losses due to burning account for 30%, while sidestream smoke, incomplete absorption and retention within the cigarette ('joint') also produce significant losses. Inexperienced and infrequent smokers absorb approximately 10-14% of the available THC whereas regular users absorb double that amount, probably because their more efficient smoking technique allows them to hold the smoke longer in their lungs. For the other major cannabinoids, the amounts absorbed range from 31% for CBD to 38% for CBN.
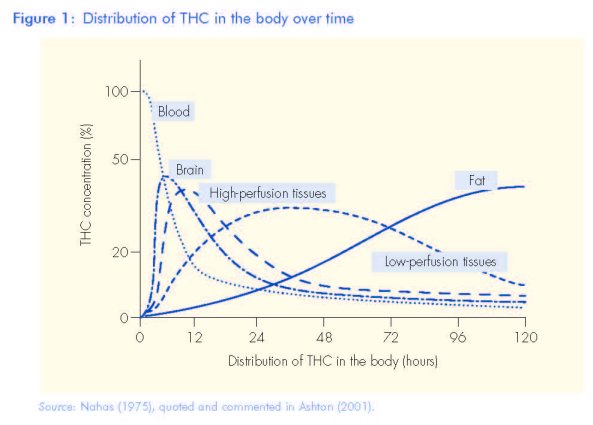
When cannabis is smoked, the effects start within seconds, reach a peak around 20 minutes and last for two to three hours (Figure 1). In contrast, if the drug is eaten, the effects are delayed and last longer, reaching a maximum about 3-4 hours after drug ingestion, and lasting for six to eight hours (Grotenhermen, 2003). After smoking, THC is detectable in the plasma only seconds after the first puff of a cannabis cigarette,
with peak plasma level being measured 3-10 minutes after the first puff. This reflects the conversion of THC to its metabolites. This metabolism takes place in the liver and involves different enzymes, some of which are inhibited by CBD, which can thus affect the metabolism of THC. THC is further metabolised to a non-psychoactive molecule, which is excreted in urine as its glucuronide, although more than 100 different metabolites of THC have been identified (Hawksworth and McArdle, 2004). Only traces of the original THC are found in urine.
Because THC is highly fat soluble (lipophilic), plasma levels of THC fall rapidly after 30 minutes. However, its many metabolites are only slowly eliminated from the body as they are stored in fatty tissues. Complete elimination may take up to five weeks. So repeated cannabis use leads to an accumulation of cannabinoids in lipid-rich tissues including the brain. THC is slowly released from fatty tissues into the bloodstream. There is, however, no simple relationship between the level of THC and its metabolites in blood and behavioural effects, such as psychomotor impairment (Agurell et al., 1986). This is because there is a delay between the subjective 'high' and THC in blood, and there are also large variations in individual psychoactive effects experienced at the same THC level in blood (see Figure 1).
The endocannabinoid system
THC and other cannabinoids act by binding to specific cannabinoid receptors found on the surface membranes of various cells located chiefly in the brain and in the immune system. Two receptors have been identified. The first cannabinoid receptor, CB1 (Matsuda et al., 1990), is expressed in the brain, in nerve cells, the reproductive system, some glandular systems and the microcirculation (Howlett et al., 2002, 2004; de Fonseca et al., 2005). The second cannabinoid receptor, CB2, is expressed in the peripheral tissues, principally in the immune system (Munro et al., 1993; Felder and Glass, 1998; Pertwee, 1999).
The discovery of these receptors — and there may be others in the body — led to the identification of a family of sendocannabinoids'. These molecules are arachidonic acid derivates which have potent actions at the cannabinoid receptors. The discovery of cannabinoid receptors and their endogenous ligand, the endocannabinoids, suggested the existence of an endogenous cannabinoid system. Subsequent elaboration of the biosynthesis, release, transport and degradation of these endocannabinoids within the body led to the realisation that they formed part of a new signalling system within the body termed the 'endocannabinoid system'. This has interactions with other neurotransmitters including gamma-aminobutyric acid (GABA), the opioid receptors and the dopamine system. The endogenous cannabinoid system seems to act as a neuromodulatory system, generally inhibiting the release of other neurotransmitters. CBD, on the other hand, does not bind to the CB receptors but may exert its sedating, hypnotic effects through other cannabinoid receptors which are believed, but not proven, to exist.
Cannabinoid receptors control cell differentiation in the developing brain. One of their most remarkable features is their high concentration within the brain, with densities 10-50 times greater than those of the classical neurotransmitter receptors, for example those for dopamine and opioids. CB1 receptors are expressed at particularly high densities in the cerebellum, hippocampus and in the basal ganglia (striatum, globus pallidum and substantia nigra). The presence of cannabinoid receptors in the hippocampus and the cortex suggested their involvement in the learning and memory process, whereas cannabinoids appear to mediate effects on motor activity, coordination and reactions through receptors in the basal ganglia and cerebellum. CB1 receptors are also found in the nucleus accumbens and frontal cortex, which is believed to account for the reinforcing effect of cannabinoids. Indeed, the endocannabinoid system controls the motivation for appetite stimuli, including food and drugs. Drugs of dependence tend to activate dopamine-producing nerve cells in the ventral tegmental area (VTA) and THC is no different because it increases dopamine release in the nucleus accumbens and prefrontal cortex.
The numerous investigations into the endocannabinoid receptor system and its interactions with other neuronal systems have resulted in a large body of scientific evidence which indicates that CB1 receptors, especially in the striatum, nucleus accumbens and the prefrontal cortex, mediate virtually all of the behavioural and neurochemical properties of THC and other cannabinoids. In particular, rewarding effects, tolerance and physical dependence have been ascribed to the brain endocannabinoid system and its interactions with the opioid, glutamate, GABA and especially the dopaminergic systems (Tanda and Goldberg, 2003). Gardner (2002) concluded that cannabinoids act on brain reward processes and related behaviours in ways that are remarkably similar to other addictive drugs. Studies with CB1 antagonists have shown the importance of these receptors in the whole phenomenon of craving. Ongoing studies highlight the significance of the endocannabinoid system in alcohol dependence, smoking cessation, weight loss, and self-administration of cocaine and opioids.
The CB1 receptor has also been identified in both male and female reproductive systems including the ovaries, the uterine endometrium, the testis, sperm, vas deferens and urinary bladder. Recent studies reviewed by Park et al. (2004) have demonstrated that marijuana, THC and other exogenous cannabinoids exert potent effects on
the endocannabinoid system in both the gonads and during pregnancy. Current understanding indicates that endocannabinoids may be critical for embryo implantation and miscarriage.
The CB2 receptor has been detected in the spleen, tonsils and thymus gland, which are the major tissues involved in immune cell production. Cannabinoids including THC — which activate these receptors (agonists) generally — suppress the functions of lymphocytes, natural killer cells, macrophages and mast cells. Roth et al. (2002) summarised knowledge concerning CB2 receptors and cells involved in the immune system. They suggest a dynamic interaction between the receptors and the immune system, particularly leucocytes. Receptor expression is markedly altered in habitual cannabis smokers and the pattern of T lymphocyte responses to THC and the resulting immunological events may explain epidemiological reports linking cannabis use to opportunistic infections, AIDS and respiratory tract cancers. Nevertheless, as Witton (this monograph) points out, the evidence is not conclusive. Roth et al. (2002) observe that the most convincing evidence of immunosuppression comes from examining
the antimicrobial activity of alveolar macrophages. Those from herbal cannabis smokers exhibit defective phagocytosis, are impaired in their ability to produce key
immunological chemicals (interleukins, tumour necrosis factor, etc.) and in their ability to exhibit effective antibacterial activity when challenged with pathogenic bacteria. Because cannabinoid receptors are not found in significant numbers in the brain stem, cannabis is not considered to be a drug with fatal overdose risks.
Bibliography
Adams, I., Martin, B. (1996), 'Cannabis: pharmacology and toxicology in animals and humans', Addiction 91: 1585-1614.
Agurell, S., Halldin, M., Lindgren, J., Ohlsson, A., Widman, M., Gillespie, H., Jollister, L. (1986), 'Pharmacokinetics and metabolism of A-9-tetrahydrocannabinol and other cannabinoids with emphasis on man', Pharmacological Reviews 38(1): 21-43.
Ashton, H. (2001), 'Pharmacology and effects of cannabis: a brief review', The British Journal of Psychiatry 178: 101-106.
Buchanan, B. E., O'Connell, D. (1998), 'Survey on cannabis resin and cannabis in unsmoked
handrolled cigarettes seized in the Republic of Ireland', Science and Justice 39 (4): 221-222.
Felder, C., Glass, M. (1998), 'Cannabinoid receptors and their endogenous agonists: review', Annual
Review of Pharmacology and Toxicology 38: 79-200.
de Fonseca, F. R., Del Arco, I., Bermudez-Silva, F., Bilbao, A., Cippitelli, A., Navarro, M. (2005), 'The
endocannabinoid system: physiology and pharmacology', Alcohol and Alcoholism 40: 2-14.
Gardner, E. (2002), 'Addictive potential of cannabinoids: the underlying neurobiology', Chemistry
and Physics of Lipids 121: 267-290.
Grotenhermen, F. (2003), 'Pharmacokinetics and pharmacodynamics of cannabinoids', Clin Pharmacokinet 42: 327-360.
Hawksworth, G., McArdle, K. (2004), 'Metabolism and pharmacokinetics of cannabinoids,' in Guy, G., Whittle, B., Robson, P. (eds), The medicinal uses of cannabis and cannabinoids, London Pharmaceutical Press, London, 205-228.
Howlett, A., Barth, F., Banner, T., Cabral, G., CaseIlas, P., Devane, W., Felder, C., Herkenham, M.,
Mackie, K., Martin, B., Mechoulam, R., Pertwee, R. (2002), 'International Union of Pharmacology
XXVII. Classification of cannabinoid receptors', Pharmacological Reviews 54: 61-202.
Howlett, A., Breivogel, C., Childers, S., Deadwyler, S., Hampson, R., Porrino, L. (2004), 'Cannabinoid
physiology and pharmacology: 30 years of progress', Neuropharmacology 47: 345-358. Matsuda, L., Lolait, S., Brownstein, M., Young, A., Bonner, T. (1990), 'Structure of a cannabinoid
receptor and functional expression of the cloned cDNA', Nature 346(6284): 561-564.
Munro, S., Thomas, K., Abu-Shaar, M. (1993), 'Molecular characterization of a peripheral receptor
for cannabinoids', Nature 365(6441): 61-65.
Musty, R. E. (2004), 'Natural cannabinoids: interactions and effects', in Guy, G., Whittle, B., Robson, P. (eds), The medicinal uses of cannabis and cannabinoids, London Pharmaceutical Press, London, 165-204.
Park, B., McPartland, J., Glass, M. (2004) 'Cannabis, cannabinoids and reproduction', Prostaglandins, Leukotrienes and Essential Fatty Acids 70: 189-197.
Pertwee, R. (1999), 'Pharmacology of cannabinoid receptor ligands (review)', Current Medicinal Chemistry 6: 635-664.
Roth, M., Baldwin, G., Tashkin, D. (2002), 'Effects of delta-9-tetrahydrocannabinol on human
immune function and host defense', Chemistry and Physics of Lipids 121: 229-239.
Tanda, G., Goldberg, S. R. (2003), 'Cannabinoids: reward, dependence and underlying neurochemical
mechanisms - a review of recent preclinical data', Psychopharmacology 169: 115-134.
| < Prev | Next > |
|---|












