The effects of 3,4-methylenedioxymethamphetamine (MDNIIA) and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain
Drug Abuse
(as published in) European Journal of Pharmacology, 128 (1986) 41-48 Elsevier
The effects of 3,4-methylenedioxymethamphetamine (MDNIIA)
and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic
systems in the rat brain
Donna M. Stone, Danese C. Stahl, Glen R. Hanson and James W. Gibb *
Department of Biochemical Pharmacology and Toxicology, 113 Skaggs Hall, University of Utah, Salt Lake City, UT 84112, U. S. A.
* To whom all correspondence should be addressed.
Received 17 March 1986, accepted 3 June 1986
The effects of two amphetarnine-like designer drugs, 3,4-inethylenedioxyamphetarnine (MDA) and 3,4-methylenedioxyinethamphetamine (MDNIA), on doparninergic and serotonergic systems in the rat brain were investigated and compared to those of methamphetarnine (METH). Like METH, single or multiple 10 rng/kg doses of either drug caused marked reductions in both tryptophan hydroxylase (TPH) activity and concentrations of 5-hydroxytryptarnine (5-HT) and 5-hydroxyindoleacetic acid, in several serotonergic nerve terminal regions. In all regions examined, the reduction in 5-HT content corresponded to the depression of TIPH activity. Unlike multiple METH administrations, which induced pronounced deficits in doparninergic neuronal markers, repeated doses of NIDA or M13MA did not alter striatal tyrosine hydroxylase (TH) activitiek or reduce striatal doparnine concentrations. A single dose of MDA or MDMA significantly elevated striatal doparnihe content; however, after repeated drug administrations dopiamine concentrations were comparable to control values. At this time, striatal levels of homovanilfic acid were significantly elevated suggesting that both drugs influence dopamine turnover. The effects of N[DA or MDMA administration in the rat brain are reminiscent of those elicited by p-chloroamphetamine, a presumed serotonergic neurotoxin.
keywords:
3,4-Methylenedioxyamphetarr~ne; 3,4-Methylenedioxymethamphetartiine; Tryptophan hydroxylase;
Tyrosine hydroxylase; Scrotonin; Doparnine
1. Introduction
MDA and MDMA are synthetic amphetamine analogs which have attracted recent public attention, as well as growing interest and concern in the scientific and medical communities. As substituted Phenylethylarnines, they are structurally related toa wide variety of other synthetic and naturally occurring compounds including amphetamine, a classical psychornotor stimulant; mescaline, a potent hallucinogen; and catecholarnines. Variations in the location and identity of substituent groups, both on the phenyl ring and the ethylamine side chain, profoundly alter the potency and ability of these compounds to elicit stimulatory or psychotomimetic effects (Anderson et al., 1978; Shulgin et al., 1969); this characteristic renders them valuable tools with which to investigate the relationship between biochemistry and behavior.
MDA produces a combination of sympathomimetic stimulation and perceptual alterations (Gunn et al., 1939), and its clinical toxicity parallels that of amphetamine (Simpson and Rumack, 1981), whereas its N-methylated derivative, MDMA ('ecstasy'), is neither a true hallucinogen nor a potent stimulant; rather, it induces a unique state of enhanced emotional and sensory awareness (Adler et al., 1985). Although both compounds have been advocated as aids to psychotherapy (Adler et aL 1985; Naranjo et al., 1967), and MDA has been evaluated as a possible anorexigenic or antidepressant agent (Smith. Kline and French laboratories, 1957, Report on clinical evaluation of SK17 No. 5 (amphedoxamine), Philadelphia, PA), there is, currently, no legitimate therapeutic use for either compound.
Recent findings by Ricaurte et al. (1985) suggest that MDA is selectively toxic to serotonergic neurons in the rat brain. These investigators found morphological evidence of nerve terminal degeneration in brain regions corresponding to those in which MLIA had produced selective long-lasting reductions in three serotonergic neuronal markers. Although the toxicity of MDMA has not previously been assessed, its high abuse liability. increasingly widespread recreational use, and structural similarity to MDA prompted the Drug Enforcement Administration to place MDMA under a temporary 'Schedule I' controlled substance classification (Lawn, 1984, I-ederal Register 50 (150), 23 118) along with MLIA, LSD and heroin.
In this study we have focused on the responses of the serotonergic and dopaminergic neurotransmitter systems to MDA and MDMA. Effects of these two designer drugs were compared to those of METH, a structural analog which is known to cause long-lasting reductions in the brain concentrations of both dopamine and serotonin and their major metabolites (Kc>da and Gibb. 1971 Ricaurte et al., 1980; Wagner et al., 1980), as ell as significant decreases in TH and TPH activities, the rate limiting enzymes for doparnine and serotonin synthesis, respectively (Hotchkiss and Gibb, 1980).
We now report that single or multiple doses of MDA or MDMA cause neurochemicai deficits in serotonergic pathways which are similar to those induced by METH; in contrast, the response of dopaminergic pathways to these agents and METH are very different.
2. Materials and methods
2 1 Drug adounixtration
Male Sprague-Davley rats weighing 200-275 g were housed 5 per cage and maintained in a temperature controlled room (26'C) with a 12 h alternating light-dark cycle. They were allowed free access to standard laboratory food and water. All drugs, as their hydrochloride salts, were dissolved in 0.9% saline and administered by subcutaneous injection; doses are expressed in terms of the free base. Subacute drug treatment consisted of 5 sequential doses at 6 h intervals of either d-METH (15 mg/kg), d]-MDA (10 mg/kg) or di-MDMA (10 mg/kg). Acute treatment consisted of a single injection of drug (MDA or MDMA, 10 mg/kg). Control animals received similar injections of saline vehicle alone.
Rats were killed by decapitation 18 h (subacute) or 3 h (acute) after the last dose (within a 3 h period at mid-day), and the brains were immechatch removed and placed on ice. The neostriata, hippocampi, and frontal cortex regions were removed bilaterally by dissection, frozen on dry ice. and stored at -70'C until assayed. One of the paired striata. hippocampi, or cortex regions from each rat was assayed for TPH and TH (striata only) enzyme activities, while the other was used in the quantitation of monoamine and monoamine metabolite concentrations.
2.2. Enzime assays
During all steps in the preparation of enzymes. reaction tubes and mixtures were kept on ice. Tissues were weighed and homogenized (1 :31) in 50 mm 4-(2-hvdroxyethyl)-1-piperazinc-N'-2ethane sulfonic acid (HEPES) buffer (Sigma Chemical Co., St. Louis, MO) pH 7,4 containing 0.2% Triton X-100 and 5 mM dithiothreitol. Homogenates were centrifuged at 27000 X g for 15 min. Duplicate 7.5 gl aliquots of the supernatant fraction from each sample were analyzed for TH activity by a tritium release assay (Nagats i et al., 1964) utilizing [31-11tyrosine as substrate. Similar aliquots were diluted to 50 btl with glass-distilled water and assayed for TPH activity using a modified "C02-trapping procedure (lchiyama et al., 1970; Sitaram and Lees, 1978) based on the TPHcatalyzed hydroxylation of carboxyl-"C-labeled tryptophan. The "CO, liberated in the presence of excess aromatic L-amino acid decarboxylase was trapped on a piece of filter paper saturated with hyamine hydroxide. Details of the assay are described by Hotchkiss et al. (1979).
2.3. Determination of monocomne and monoamine nietabolite concentrations
Ti ssue levels of dopamine (DA) and its primary metabolites. 3,4-dihydroxyphenylacetic acid iDOPAC) and homovanillic acid (HVA ). and 'hydroxytryptamine (5-HT) and its major rnelabolite, 5-hydroxyindoleacetic acid (5-HIAA), were measured by h i gh- performance liquid chromatography (HPLC) with electrochemical detection. Tissues were weighed and homogenized in 0.3-0.5 m] mobile phase buffer containing 0.15 M fflonochloroacetic acid, 2.0 mM disodium EDTA and 25 ing/1 1-octanesulfonic acid in 12.5% methanol at pH 2.9. After centrifugation at 4080 x g for 15 min the supernatant fractions were filtered through a 0.2 jam microfilter system (Bioan. lytical Systems. Inc., West Lafavette, IN). Fifty microliter volumes were injected by a WISp`rM automatic sample processor (Millipore Corp., Miliford, MA) onto a 3 gm reverse phase Micro%orb C, column (Rainin Instrument Co., Woburn, MA). Samples were run on a LC-154 liquid chromatograph equipped with a model LC-4B amperometric detector (Bioanalytical Systems, Inc.). Tle detector potential was set at +0.73 V. Monoammes and their metabolites were quantitated by measurement of peak heights and comparison with those of standards of known concentration prepared in mobile phase buffer.
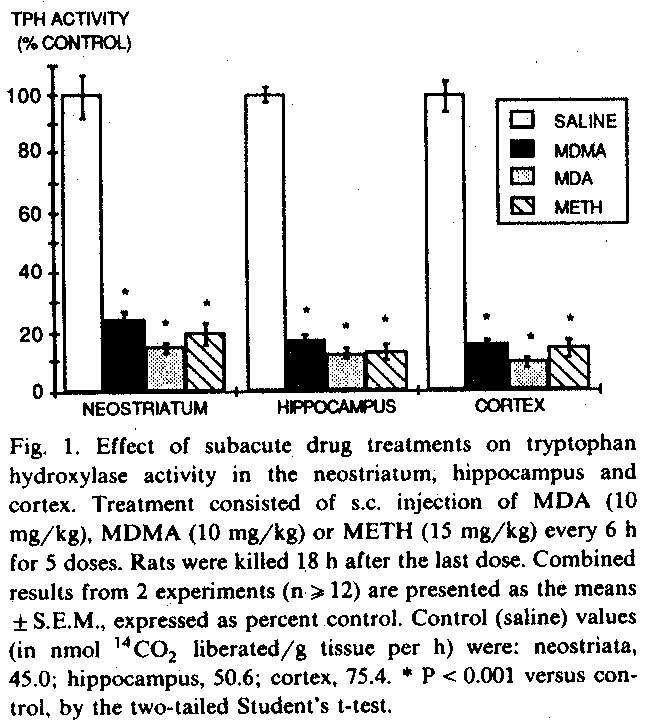
3. Results
3. 1. Subacule effects of MDA, MDMA and METH
Eighteen hours after termination of subacute treatment with MDA ' MDMA or METH, TPH activity was markedly depressed in all brain regions examined (fig. 1). After MDA treatment, remaining enzyme activity ranged from 14% of control in the neostriatum to 9% of control in the cerebral cortex. Slightly less dramatic reductions were consistently observed after MDMA treatment (remaining TPH activity ranged from 25% of control in the neostriatum to 15% of control in the cerebral cortex). METH-induced decreases in TPH activity were comparable to those induced by either MDA or MDMA.
Figure 2 presents the results of HPLC determinations of 5-HT and 5-1-11AA concentrations in selected brain regions following subacute drug treatment. Levels of 5-HT and 5-HIAA were de creased to less than 30% of controls by both MDA and MDMA in all regions examined. These results correlate with the regional depression of TPH activity induced by either drug. METH treatment resulted in reductions of a similar magnitude; values were consistent with previous reports (Hotchkiss and Gibb, 1980).
Of the three compounds only METH significantly affected neostriatal TH, as evidenced by a 56% reduction in enzyme activity (fig. 3); this response to subacute METH treatment has been characterized previously (Koda and Gibb, 1973).
MDA or MDMA, even when administered in higher doses (15 mg/kg, data not shown), did not significantly alter TH activity.
The effects of drug treatments on DA and DA metabolite levels are depicted in fig. 4. Although repeated MDA or MDMA administrations did not significantly alter neostriatal DA or DOPAC concentrations, both drugs significantly increased the levels of HVA in this region, to 124 and 116% of controls, respectively. In contrast. neostriatal DA, DOPAC and HVA were all significantly decreased after METH treatment, to 26, 39 and 50% of controls, respectively. The METH-induced reduction in DA content was consistent with the decrease in TH activity observed in this region. lit the two other brain regions examined, control levels of DA and its major metabolites were too low for accurate measurement.
3.2. Acute effects of MDA and MDMA
Three hours after a single injection of MDA or MDMA, neostriatal TPH activity was reduced to less than 50% of control (fig. 5). Significant reductions occurred also in other brain regions (for MDA the values were: hippocampus 60% of control, cortex 49% of control; for MDMA: 52 and 30% of control in the hippocampus and cortex, respectively). Corresponding decreases in 5-HT and 5-HIA-A levels were observed in all regions (fig. 6).
Although neostriatal TH activity was not affected by acute administration of either MIDIA or MDMA (fig. 5), DA levels were significantly elevated after a single injection of either drug (fig. 7). However, the effects of the two drugs on neostriatal concentrations of DA metabolites differed. Concentrations of DOPAC were decreased following MDA treatment, while HVA levels remained unchanged. In contrast, MDMA treatment did not significantly alter DOPAC concentrations, but significantly increased those of HVA (fig. 7).
4. Discussion
The present study provides evidence to suggest that high doses of either MDA or its N-methylated derivative, MDMA, are selectively 'toxic' to scrotonergic neurons in the rat brain. Single or multiple doses of either drug dramatically reduced both 5-HT concentrations and TPH activities in three scrotonergic nerve terminal regions. Histological examination has revealed MDA-induced nerve terminal damage in hippocampal and striatal regions of the rat brain (Ricaurte et at., 1985); the similarities of the neurochenucal deficits resulting from both NIDA and MDMA treatments indicate that, like MDA, MDMA is also neurotoxic. Two mechanisms which are thought to be involved in the serotonergic effects elicited by other amphetamine analogs, namely drug-induced 5-HT release, and inhibition of 5-HT reuptake (SandersBush and Massari, 1977; Wong et al., 1973), ntight contribute to the lowered serotonin levels observed after MDA or MDMA treatments. In this regard, both MDA and MDMA are potent inducers of 5-HT release in vitro (Nichols et al., 1982).
The neurochemical alterations induced by MDA or MDMA in the rat brain are strikingly similar to those observed following the administration of p-halogenated amphetamine derivatives such as pchloroamphetamine (PCA). A single dose (10 mg/kg) of PCA in the rat causes rapid and longlasting reductions (up to 4 months) in the levels of brain scrotonin and 5-HIAA (Sanders-Bush et al., 1972). Corresponding reductions in TPH activity, as well as decreases in the number of high affinity serolonin uptake sites in brain slices and synaptosomal fractions, have been reported (Carlson, 1970; Wong et al., 1973). These findings, in conjunction with histological evidence of nerve cell degeneration in the scrotonin-rich B-9 cell region of the mesencephalon (Harvey et al., 1975), and ultrastructural evidence of degenerated axon terminals in the striatum (McGeer et al., 1975), have led several investigators to suggest that PCA, or some metabolite, exerts a specific neurotoxic action on serotonergic pathways. Like MDA and MDMA, PCA is not toxic to catecholamine new rons.
Although PCA has been extensively studied, the precise mechanism of its selective neurotoxic action is, as yet, unknown. However, its ability to lower brain content of 5-hydroxyindoles is blocked by fluoxetine (Fuller et al., 1975), an inhibitor of sercitonin uptake, suggesting a requirement for transport into serotonergic neurons. Recently, a brief communication (Gehlert et al., 1985) reported that the serotonergic deficits induced by MDMA can be blocked by co-administration of the serotonin reuptake inhibitor citalopram, suggesting that the neurotoxic mechanism of MDMA may be sintilar to that of PCA. However, this report also indicated that MDMA was probably not concentrated in nerve terminals. Additional studies are required to determine the molecular events responsible for the serotonindepleting effects of these agents.
Unlike METEL neither MDA nor MDMA are toxic to DA neurons. However, the alterations of DA and DA metabolite levels after single or multiple drug injections indicate that these agents do affect the dopaminergic system Three hours after a 10 mg/kg dose of either drug, DA levels were significantly elevated, although TH activity was unchanged (figs. 7 and 5. respectively). However. this effect on DA concentrations was not o~ed after multiple drug administrations (fig. 4). Such an initial. transient elevation of striatal DA has been observed following acute administration of other amphetamine analogs, including amphetamine (Kuczenski, 1978), METH (Koda and Gibb, 1973; Kogan et aL 1976), and PCA (Leonard, 1976), and could reflect either an increase in DA synthesis, a decrease in DA catabolism or a conihination of both actions. Since DA synthesis is thought to be regulated by TH. the enzyme which catalyzes the rate-linuting step in catecholamine biosynthesis, an activation of enzyme by some mechanism undetectable in our TH assay could account for the observed increase in DA concentration; possible mechanisms include a decreased end-product inhibitien (due, perhaps, to drug-induced DA release, see below) or an alteration of the enzyme molecule resulting in either an increased affinity of enzyme for the pteridine cofactor (Zivkovic et al., 1974), or a decreased affinity for the catechol inhibitor (Neff and Costa,
1966). Decreased DA catabolism could be attributed to monnamine oxidase inhibition, an effect observed with both MDA (Mann and Quastel, 1940) and MDMA (Fellows and Bernheim, 1950). Although it is conceivable that such a mechanism plays a role in the acute effects of these drugs, the MDMA-induced elevation of FIVA (see fig. 7) appears inconsistent with such an interpretation.
Regarding the latter phenomenon, previous experiments in our laboratory have revealed an increase in the concentration of striatal FIVA within the first few hours after acute METH treatment (unpublished observations); similar effects have been observed after a single dose of amphetamine (Jori and Bernardi, 1969). An elevated brain EIVA content is often interpreted as evidence of enhanced DA turnover consequent to drug-induced DA release, and, indeed, acute amphetamine administration increases both the synthesis (Schwartz et al., 19W Uretsky and Snocigrass, 1977) and release (Azzaro and Rutledge, 1973; Chitieh and Moore, 1975) of DA. The leveis of HVA were significantly elevated following subacute treatment with either MDA or MDMA (fig. 4) suggesting, perhaps. that these two drugs also cause DA release.
The response of the catecholaminergic system to acute administration of MDA differed from that elicited by acute administration of MDMA, as evidenced by the inconsistent effects of the two drugs on striatal HVA concentrations (fig. 7). In this regard. it is of interest to note that MDA and MDMA have been proposed to act by different mechanisms. Evidence derives from studies performed in rats demonstrating a lack of cross toler
ance between the two agents (Nichols et al., 1982); moreover, optical requirements for the central activity of MDA were reversed upon methylation (Anderson et al., 1978). This latter finding argues against the possibility that MDMA is activated in vivo by way of demethylation.
The ability of these drugs to influence the dopaminergic system might be an important determinant of long-term effects. Previous investigations in our laboratory have demonstrated that agents which preferentially influence doparninergic activity, i.e. haloperidol, a DA receptor antagonist; a-methyl-p-tyrosine, an inhibitor of DA synthesis; or amfonelic acid, a specific DA uptake blocker, prevent the effects of METH on both dopaminergic and serotonergic neurotransmitter systems (Schrinidt et al., 1985). It appears that the METH-induced alterations in the dopaminergic system are causally linked to changes in the serotonergic system, and that DA is the common mediator for toxic effects in both systems. Due to the similarity of the serotonergic effects elicited by METH. MDA and MDMA, it would be of interest to determine whether the 'neurotoxic' effects of the latter two agents are also linked to the dopaminergic system. This possibility is currently being explored in our laboratory.
In summary, our results demonstrate that both MDA and its N-methylated derivative. MDMA, cause selective neurochemical deficits in serotonergic pathways in the rat brain; these effects are observed after a single injection of either drug and become more pronounced with multiple drug administrations. In addition, we have shown that both MDA and MDMA appear to influence the dopaminergic system (at least initially), and this action may play an important role in their shortterm behavioral as well as lone-term biochemical
Acknowledgements
This research was supported by USIPHS Grants DA 00869 and GM 07579. The authors wish to thank the National Institute of Drug Abuse for the gifts of d-METH hydrochlo ride, dl-MDA hydrochloride and diMDMA hydrochloride
Adler J., R Abrahamson, Katz & Hager 1985, Getting high on 'ectsasy', Newsweek April 15. 96.
Anderson, G.M., III, G, Braun, U, Braun, D.E. Nichols and A,T. Shulgin, 1978. Absolute configuration and psychotornimetic activity, in: Quasar Research Monograph 22
Azarro A.J. and C.O. Rutledge, 1973, Selectivity of release of norepinephrine, dopamine and scrotonin by amphetamine in various brain regions, Biochem. Pharmacol. 22, 280L
CarIsson, A_ 1970, Stmeural specificity for inhibition of '4C. 5-hydroxytryptamine uptake by cerebral slices, J. Pharm, Pharmacol. 22, 729.
Chitich, CC and K.E. Moorc. 1975, d-Amphetanune-induced release of newly synthesized and stored dopanune from the caudate nucleus in vivo, J. Pharmacol. Exp. Ther. 192, 642.
Fellows, E.J. and F. Bernheim, 1950, The effect of a number of arytalkylamines on the oxidation of tyramine by amine oxidase, J. Pharmacol. Exp. Ther, 100, 94~
Fuller. R.W_ KY. Perry and B.B. Molloy. 1975, Effect of 3-(p-trifluoromethyl-phenoxy)-N-methyl-3-phenylpropyiamine on the depiction of brain scrotonin by 4-chloroamphetamine, L Pharmacol. Exp. Ther. 193, 796.
Geldert, D.R.. C.L Schmidt, L. Wu and W. Lovenherg, 1985. Evidence for specific meihylenedioxymethamphetamine (ecstasy) binding sites in the rat brain, European J. Ph.r..wl. 119. 135,
Gunn, IA--- M.R, Gurd and I. Sachs. 1939. The actions of some amines related to adrenaline: ~thoxyphenyli~pr~ pyi..i..s, J, Physi.l. 94, 485.
Harvey, J.A., S.E. MeMasier and L.M. Yunger. 1975. pChloroamphetamine: selective neurotoxic action in brain. Science 187, 84).
Hoichkin. A and LW Gibb, 1980, Long-term effects of multiple doses of methamphotamine on tryptophan hydroxylaw and tyrosine hydroxylase activity in rat brain, J. Ph.r..~ol. Exp. -Rc,. 214, 257,
H.t.hkiss, A, M M-gism and 3.W. Gbh, 1979, The 1..g-tcreffect of multiple doses of ~thamphelamine on ncostriatal tryptophan hydroxylase, tyrosine hydroxylasw. chofinc acetyliransferase and glutamate deearboxyiasc activities. Life Sc.. 25. 1373
lchiyama. A_ S. Nakamura, J. Nishiruka and 0. Haymshi. 1970. Ewymatic studies on the biosynthesis of wrotonin in mammalian brain, J. Biol. Chern. 245. 1699,
Jon, A. and D. Bcmarch, 1969. Effect of amphetamine and ~pheamine-like drugs on homovanilbe acid concentration in the brain. J. Pharm, Pharmacol. 21. 694.
Koda, L.Y. and 1 W. Gibb. 1973, Adrenal and striatal tyrosinc hydroxylasc, activity after methamphetarnine, J. Phamawl. Esp, Ther. 185, 42.
Kogan, F.J_ W.K Nichols and J,W. Gibb, 1976. influence of methamphetarnine on mgral and striatal tyrosine hydroxy]axe activity and on striatal cloparnine levels, European J. Pha-col. 36. 363.
Kucrenski, R_ 1978, Biphasic effekis of amphetamine on stratal doparrum, dynamics, European J. Phamawl. 46, 249.
Lconard. WE,, 1976, Acute and chronic effmts of 4-chloroamphetamine on monoamine metabolism in the rat brain. Psychopharmacologia (Berlin) 46, 1,
Mann, RJ.G. and J.11. Quastel, 1940, Berizedrine (fl-phenyli~ propylaminc) and brain metabolism. Biochern. 3. 34, 414,
MeGmr, E.. T. Hatton and P. MeGeer. 1975, Electron microscopic studies on p-chloroamphetamine-indmed degeneration of striatal synaptosomes, Neurosci. Abstr. 1, 198,
Nagatsu. T_ M. Levitt and S. Udenfriend, 1964. A rapid an(t simple radioassay for tyrosine hydroxylaw activity, Anal Biochern. 9, 122,
Naranjo, C_ AT. Shulgin and T. Sargent, 1967, Evaluation of 3.4-methylenedioxyamphetamine (MDA) as an adjunct t, psychotherapy, Med. Pharmacol. Exp. 17, 359.
Neff. N.H, and E. Costa, 1966, The influence of monoamine oxidaw inhibition on catecholamine synthesis, Life Ski, 5, 951,
Nichols, D.E.. D.H. Lloyd, A.1 Hoffirnan, M.B. Nichols and G.K.W, Yim, 1982, Effects of certain hallucinogenic amphetamine analogues on the release of ['H]"rotonin from rat brain synaptosomcs, J. Mod. Chem. 25, 530.
Ricaurtc, G_ 0. Bryan, L. Strauss, L, Sciden and C, Schuster. 1985, Hallucinogenic amphetamine selectively destroys brain scrotonin nerve terminals. Science 229, 986.
Ricaurtc, G.A., C.R. Schuster and L.S. Sciden. 1980, Long-term effects of repeated methylamphetannine administration on dopamine and scrotonin neurons in the rat brain: a regional study, Brain Res. 193, 153.
Richards, R.N.. 1972, Experience, with MDA, Can. Med~ 3 106, 257.
Sanders-Bush, E_ I.A. Bushing and F Suiscr, 1972, Long-term effects of p~hlor~amphetamine on tryptopiian hydroxyl---activity and on the levels of 5-hydroxyt~ptamine and 5-hydmxyindoleacctic acid in brain. European J Pharma. c.1 20, 385,
Sanders-Bush, E. and L Massan, 1977, Actions of drugs that deplete wrotonin. Fed. Proc, 36 (8). 2149.
Schmidt. C.I. LK. Riter. P K, Somusila. GR. Hanson and LW. Gibb. 1985. R.1c of d.p..i.c in the effects of methamphotanune. J.Pharmacol. Exp. Ther 233, 539.
Sch-cu, R.D.. N.J. U,ctsky and J.R. B,a.ch,.e, 1980. The relationship between the stimulation of dopamine synthesis and reicaw produced by amphetamine and high potassium in sinatal slices. J. Neurmhem, 35 (5). 1120~
Shulgin. AT.. T, Sargent and C. Naranjo. 1969. Structure-ac. uvity relationships of one-ring psychotomimeties. Nature 221. 537.
Simpson, D.L. and B H. Rumack, 1981, Methylened, oxyamphetamne, clinical description of overdow, death. and review of pharmacology. Arch, Int. Med. 141. 1507.
Sitaram. BA, and G,J. Lers, 1978. Diurnal rhythm and tryptophan hydroxylasc in the pineal gland of the rat, J Neuroclic., 31. 1021.
Uretsky, N.J. and R. Snodgram, 1977, Studies on the mcchanism of stimulation of depamine synthesis by amphetamine
ut ],-, J. Pha,..-i. Exp. The, 202. 565.
Wagner, &C,, G.A. Ricaurte. L.S. Sciden. C.R. Schuster. J Miller and J. Wuticy, 1980, Long-lasting depictions of struital doparnme and loss of dopamint uptake sites followmg repeated administration of methamphearnine. Brain Res. 181. 151. Wong. DT--- LS. Horng and R.W. Fuller, 1973. Kinetics of scrotonin accumulation into synaptosomes of rat brain effects of amphetamine and chloroamphetamines, BiochePh.r.-1. 22, 311.
Zivkovic. B_ A. Guidout and E. Costa, 1974. Effects of neuroleptics on striatal xvrosint hydroxylaw: changes in affinity for the oteridine cofactor. Mol. Pharmacol. 10, 727.
Last Updated (Monday, 20 December 2010 19:52)












