Serotonin NeurotoxicitY after (±)3,4Methylenedioxymethamphetamine (MDMA; "Ecstasy"): A Controlled Study in Humans
Drug Abuse
NEUROPSYCHOPHARMACOLOGY 19,94-VOL. 10, NO. 2
Serotonin NeurotoxicitY after (±)3,4Methylenedioxymethamphetamine (MDMA; "Ecstasy"): A Controlled Study in Humans
Una D. McCann, M.D., Alison Ridenour, B.S., Yavin Shaham, Ph.D., and George A. Ricaurte, M.D., Ph.D.
From the Unit on Arodety and Affective Disorders (UDM), Biological Psychiatry Branch, National Institute of Mental Health, Bethesda, Maryland, Department of Neurology (AR, GAR), Francis Scott Key Medical Center, Johns Hopkins Medical Institutions, Baltimore, Maryland, Department of Psychology (YS), Concordia University, Montreal, Quebec, Canada.
Address correspondence to: Dr. Una D. McCann, Unit on An)dety and Affective Disorders, BPB, National Institute of Mental Health, Building 10, Room 3S-239, 9000 Rockville Pike, Bethesda, Maryland 20892.
key words: Amphetamine; Serotonin; Prolactin; Neurotoxicity; Personality; Drug abuse
(±)3,4-Methylenedioxymethamphetamine (MDMA; "Ecstasy"), an increasingly popular recreational drug, is known to damage brain serotonin 5-hydroxytryptamine (5-HT) neurons in experimental animals. Whether MDMA is neurotoxic in humans has not been established. Thirty MDMA users and 28 controls were admitted to a controlled inpatient setting for measurement of biologic and behavioral indexes of central 5-HT function. Outcome measures obtained after at least 2 weeks of drug abstinence included concentrations of monoamine metabolites in cerebrospinal fluid (CSF), prolactin responses to L-tryptophan, nociceptive responses to ischemic pain, and personality characteristics in which 5-HT has been implicated (i.e., impulsivity and aggression). Subjects with a history of MDMA exposure had lower levels of CSF 5hydroxyindoleacetic acid (the major metabolite of 5-HT) than controls (p = .001). Although they resembled controls in their prolactin response to Ltryptophan and their response to ischemic pain, MDMA users had lower scores on personality measures of impulsivity (p = .004) and indirect hostility (p = .009). The CSF findings suggest that 5-HT neurotoxicity may be a potential complication of MDMA use. Further, differences in personality support the view that 5-HT systems are involved in modulating impulsive and aggressive personality traits. Additional studies of MDMA-exposed individuals are needed to confirm and extend the present findings. Such studies could help elucidate the role of 5-HT in normal brain function as well as in neuropsychiatric disease states. [Neuropsychopharmacology 10:129-138, 19941
(±)3,4-Methylenedioxymethamphetamine (MDMA, 'Tcstasy") is a synthetic analog of amphetamine and mescaline that has emerged as a popular recreational drug of abuse (Peroutka 1987; Anon 1991; Henry et ad. 1992), and has recently been characterized as the drug of choice for use in large organized social settings (Randall 1992). Although MDMA is considered safe by most users (Eisner 1989; Randall 1992), there is compelling preclinical evidence that NMMA produces toxic effects on brain serotonin (5-hydrox~ryptamine, 5HT) neurons in animals (Stone et al. 1986; Schmidt 1987; Commins et al. 1987; Battaglia et a]. 1987, Ricaurte et al. 1988a; O'Hearn et al. 1988). Furthermore, there is evidence that in nonhuman primates, the neurotoxicity of MDMA is prolonged (Insel et al. 1989) and possibly permanent (Ricaurte et al. 1992). Because the dose of MDMA that damages 5-HT neurons in monkeys is dose to that typically taken by recreational users (Ricaurte et al. 1988b), and because the dose-response curve for MDMA neurotoxicity in the primate is steep (Ricaurte et al. 1988a), there is growing concern that MDMA neurotoxicity may generalize to humans.
At present, there are no direct methods for detecting serotonergic neurotoxicity in the living human brain. Consequently, MDMA's neurotoxic potential in humans can only be evaluated indirectly, by measur~ ing the concentration of 5-hydroxyindoleacetic acid (S-HI.AA) in cerebrospinal fluid (CSF). Studies in monkeys indicate that CSF 5HIA.A can be used to detect serotonergic damage produced by 1ADMA, but that decreases in spinal CSF 5-1i1AA underestimate the extent of serotonergic damage in the brain (Ricaurte et al. 1988c). Only two studies have measured CSF 5-1i1AA in humans in an effort to screen for possible MDMA neurotoxicity. One of these found reductions in CSF 5HIAA (Ricaurte et al. 1990), but the other did not (Peroutka et al. 1987). Given the large number of physiologic and environmental factors that can potentially influence levels of 5-HIAA in CSF (Post et al. l~80), the different findings of these two studies are not surprising, because neither study was conducted in a controlled setting.
The purpose of the present study was to measure CSF 5-HIAA in a cohort of MDMA users under controlled conditions, and to determine whether MDMA neurotoxicity, if evident in humans, was associated with changes in functional domains in which 5-HT has been implicated. These domains include neuroendoerine function, pain, and certain personality traits such as impulsivity and aggression.
METHODS
Subjects
Fifty-eight subjects participated in the study: 30 experimental (MDMA exposed) subjects and 28 controls. The demographics of the study groups are shown in Table 1 and discussed below (see Results). The MDMA subjects were self-referred; they called one of the investigators (GAR) after learning about ongoing MDMA research at the Johns Hopkins Medical Institutions. Controls were either referred by MDMA subjects or recruited from the greater Baltimore/Washington, DC metropolitan area. All participants were screened first for eligibility in telephone interviews. For inclusion in the MDMA group, individuals had to be in good health, they had to have used MDMA on at least 25 occasions, and they could not have any of the exclusionary criteria of MDMA exposure, although prior use of other drugs was allowed, because most MDMA users had also used other drugs at some time. Information about MDMA use was obtained in several ways: (1) a preliminary telephone interview; (2) an MDMA questionnaire that asked about the number of times MDMA had been used, the usual amount of MDMA taken, the frequency of MDMA use, the last time MDMA had been used, and the highest dose of MDMA ever taken; (3) a standardized drug history questionnaire; and (4) the Scheduled Interview for DSM-IUR (Spitzer and Williams 1982). Characteristics of MDMA use are listed in Table 2, and characteristics of drug exposure other than MDMA are listed in Table 3. Exclusionary criteria for both groups included past or present major medical illness (neurologic, renal, endocrine, or hematologic), pregnancy, positive human immunodeficiency vir-us status, history of psychosis, current major depressive disorder, or current alcohol or drug dependence. All subjects agreed to refrain from any recreational drug use for at least 2 weeks prior to the study and understood that they would undergo a urine and blood drug screen upon arrival at the clinical research center. Subjects who passed the initial telephone screen underwent further screening at the time of inpatient adndssion. Potential subjects were given a detailed physical and neurologic examination, a structured psychiatric interview using the Scheduled Interview for DSM-III-R (Spitzer and Williams 1982), and laboratory tests including a blood chemistry panel, complete blood count, platelet count, urinalysis, urine drug screen, and human immunodeficiency vir-us screen. Subjects admitted to the clinical research center were maintained on a low monoan-dne diet for the duration of the study. The study was approved by the local institutional review board.
Outcome Measures
CSFMonoamine Metabolites. Lumbar punctures were performed as described (Ricaurte et al. 1990) between 8 Am and 10 AM on the third moming of the subjects' stay at the clinical research center, after an overnight fast and complete bed rest. Concentrations of monoarrune metabolites in CSF were determined by highperformance liquid chromatography coupled with electrochemical detection using the method of Kilpatrick et al - (1986) for 5-HIAA and homovanillic acid (HVA), and the method of Sharpless (1986) for MHPG. Samples of CSF from control and MDMA subjects were processed and assayed in tandem without awareness of the drug condition of each subject.
Prolactin Responses to L-Tryptophan. The prolactin response to Ltryptophan, which is thought to provide a measure of central 5-HT function in humans (Price et al 1990), was used to evaluate central 5-HT function in MDMA users. Neuroendocrine challenge with L-tryptophan was performed following the protocol of Price et al. (1990) with minor modification. Briefly, L-tryptophan (7 g) dissolved in 500 ml 0.45% normal saline was iriffised through an indwelling intravenous catheter over a 20-minute period and blood for prolactin was collected through the catheter 15 and 0.5 minutes before and 30, 40, 50, 60, 70, 90, and 120 minutes after L-tryptophan administration. Values from -15 and - 0 .5 minutes were averaged to provide basal prolactin concentrations. Peak change scores were determined by subtracting the baseline value from the highest prolactin value after L-tryptophan infusion. The prolactin area under the curve was calculated using the trapezoidal rule. Serum prolactin was determined by radioimmunoassay (Nichols Institute Diagnostics, San Juan Capistrano, CA).
Pain Measurements. Because 5-HT iias been implicated in central pain (see Richardson 1992) and because impairments in 5-HT function have been associated with alterations in nociception (see Messing and Lytle 1977), pain measures were also examined in MDMA users. Nociceptive function was assessed by the submaximum-effort tourniquet technique for inducing ischerrdc pain in humans (Smith et al. 1966). Three pain measures were obtained: (1) the subjects first self-rating of pain was used as a measure of pain "sensitivity;" (2) the time from the start of the test until the subject chose to abort testing was used as a measure of a subject's pain "endurance" (with a maximum score of 20); and (3) the total pain score (the sum of all the values taken at 30-second intervals for the duration of the test) was used as a measure of a subject's pain "tolerance."
Personality Assessments. Decreased CSF 5-H[AA levels have been found in individuals with impulsive and hostile personality traits (Linnoila et a]. 1983; Roy et al. 1988 Coccaro et al 1992. To determine whether MDMA users had alterations in these personality traits, subjects were asked to complete the Multidimensional Personality Questionnaire (Tellegen 1982), the Buss Durkee Hostility Inventory (Buss and Durkee 1957), and the Eysenck Personality Questionnaire (Eysenck 1976).
Statistical Analysis
In CSF studies, analysis of covariance was initially used to analyze the concentrations of monoarnine metabolites in the CSF of MDMA and control subjects. Age and height were used as covariates in these analyses because these factors have been reported to influence CSF monoamine metabolite levels (Bowers and Gerbode 1968; Gottfries et al. 1971; WodeHelgodt and Sedvall 1978; Stanley et al. 1985). In addition, it has been reported that the season of the year (i.e., summer, winter, spring, or fall) can influence CSF monoarrdne metabolite levels (Brewerton et al. 1987). For this reason, the CSF data were also analyzed with a 2 x 4 (group x season) analysis of covariance (ANCOVA), again covarying for age and height. Because the raw data suggested a possible effect of gender-on CSF monoamine metabolite levels, the CSF data were further analyzed with a 2 x 2 (group x gender) ANCOVA, with age and height as covariates.
In functional studies, analysis of covariance was used to analyze the prolactin response to L-tryptophan, using age, basal prolactin levels, and plasma tryptophan levels as covariates. Pain study results were analyzed with a 2 x 2 (group x gender) analysis of variance (ANOVA), and personality measures were analyzed with a multivariate 2 x 2 ANCOVA, with age and education as covariates. For the personality measures, the Bonferroni method for computing multivariate intervals was used.
In the case of either significant main effects of group or significant group x gender interactions, post-hoc comparisons were performed using Duncan's multiple range test. Correlations were assessed by Pearson's product moment. All tests were two tailed; significance was set at p < .05.
RESULTS
Demographics
Control and experimental groups were well matched for age, height, weight, and relative proportion of males and females in each group (Table 1). Although 1ADMA subjects were slightly older than controls, ANOVA indicated that the age difference was not statistically significant (F11,54] - 2.31, p = .13). There were also no significant socioeconomic, ethnic, racial, or occupational differences between the two groups, although a 2 x 2 (group x gender) ANCOVA with age as a covariate revealed a significant group x gender interaction for education (F[1,531 = 4.4, p = .04), with post hoc tests indicating that control males had more years of education than MDMA males (Table 1).
CSF Monoamine Metabolites
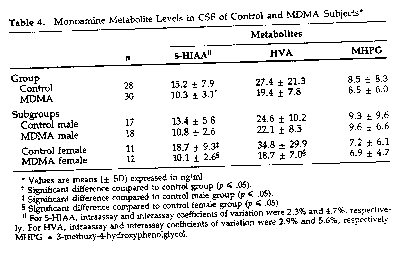
5-HLAA. Concentrations of CSF 5-HIAA in control subjects in this study (15.2 ± 7.9 ng/n-d [mean ± SD]; range: 4.8 to 37.3 ng/ml) were within the range of concentrations reported for control subjects in other studies (Post et al. 1980; Hildebrand et al. 1990; Ben Menachem et al. 1989). A one-way ANCOVA using age and height as covariates revealed that MDMA users had significantly lower levels of CSF 5-EILAA than controls (15.2 ± 7.9 ng/rrd in controls versus 10.3 ± 3.1 ng/rrd in MDMA subjects, F[1,541 - 11.6, p - .001). Because the raw data suggested a possible effect of gender, the results were further analyzed using a 2 x 2 (groups x gender) ANCOVA, again with age and height as covariates. This analysis also revealed a significant main effect of group (F11,52) = 14.9, p < . 001), as well as a significant group x gender interaction (F[1,52] = 5. 1, p < .03). Post hoc comparisons indicated that, within the M_DMA group, reductions in CSF5-HIAA were greater in females than in males (46% versus 20%), and that within the control group, males had lower levels of CSF 5-MAA than females (Table 4). The latter observation is in keeping with previous findings of others (WodeHelgodt and Sedvall 1978; Post et a]. 1980; Roy et al. 1988).
As in other studies (Wode-Helgodt and Sedvall 1978; Stanley et al. 1985), a significant negative correlation was found between height and CSF 5-H]AA in control subjects (r = -.42, p = .03). In MDMA subjects, this negative correlation was absent (r = .18, p = .34).
Cerebrospinal fluid 5-HIAA levels were negatively correlated with the number of MDMA exposures (r = -0.2), but the correlation was not statistically significant (p = .33). Cerebrospinal fluid 5-HIAA levels were not significantly correlated with the duration of MDMA use, frequency of MDMA use, or the time since last MDN1A exposure.
A 2 x 4 (group x season) ANCOVA with age and height as covariates did not show a significant effect of season, or a groupby-season interaction on CSF 5-1-HAA (F[3,50] = .89, p = .45). Comparable number of control and MDMA subjects were examined during each season of the year.
HVA. Concentrations of 1-1VA in the CSF of control subjects in this study (27.4 ± 21.3 ng/m] mean ± SD; range 8.1 to 117.9 ng/nil) were also within the range of concentrations reported for control subjects in other studies (Post et al. 1980; Hildbrand et al 1990; Ben Menachem et al 1989). A 2 x 2 (group x gender) ANCOVA with age and height as covariates revealed a significant effect of group (F[1,521 = 69, p = .01), as well as a significant group x gender interaction (F11,52] = 4.3, p = .04). Posthoc comparisons indica,,ted that NMMA females had lower levels of CSF HVA than control females but that levels of CSF HVA in control and MDMA males were not significantly different (Table 4).
MPHG. There were no significant effects of group (F[1,541 = .01, p = .9) or gender J[1,541 = .5, p = .5) on CSF MHPG levels (Table 4).
Prolactin: Basal Level and Response to L-Tryptophan
A 2 x 2 (group x gender) ANCOVAwith age as the covariate revealed a significant effect of gender on basal prolactin levels (F[1,46) = 28.5, p < .001). Post-hoc comparisons indicated that females had higher basal prolac:tin levels than males, and that basal prolactin levels did not differ between control and MDMA subjects (Table 5).
A 2 x 2 (group x gender) ANCOVA with age and basal prolactin level as covariates showed that after L-"tophan infusion, females had greater peak increases in prolactin levels than males J[1,451 = 8.2, p = .006), and that females also had larger aseas under the prolactin response curve (AUC) (F[1,45] = 5.6, p = .02) than males. No significant drug effects were found for either of the prolactin responses (peak prolactin change or prolactin AUC, Table 5).
Determination of plasma tryptophan levels before and after L-tryptophan infusion showed that the peak change in plasma tryptophan level was positively correlated with the peak change in plasma prolactin (r = .33, p = .02) and the prolactin AUC (r = .41, p = .003), When the 2 x 2 (group x gender) ANCOVA was repeated including the change in plasma tryptophan level as an additional covariate (other covariates were age and basal prolactin), the gender effects on the prolactin responses to L-tryptophan were still apparent (for peak prolactin change F[1,44) 5.9, p = .02 and for prolactin AUC F[1,441 = 4. 1, p .05), suggesting that the greater prolactin response in women is not solely related to greater peak changes plasma L-tryptophan levels.
Pain Measures
Pain Sensitivity (Initial Pain Scare). A 2 x 2 (group x gender) ANOVA showed a main effect of gender (F[1,541 = 5.7, p = .02) on pain sensitivity, with no effect of group, and no group x gender interaction. Post hoc comparisons indicated that females in both the control and MIDMA groups had higher initial pain scores (pain sensitivity) than males (Table 6).
Pain Endurance (Length of Time Pain Was Endured or Time Before Subjects Found Pain Unbearable. There were no significant effects of group or gender on pain endurance.
Pain Tolerance (Total Pain Score, See Methods). A 2 x 2 (group x gender) ANOVA also revealed an effect of gender on pain tolerance (nl,54] = 4.9, p = .03), with females having lower total pain scores than males (Table 6).
Correlation analysis revealed a negative correlation between pain sensitivity and pain endurance (r = - 0.62, p <.001) and pain tolerance (r = -0.46,p<.001) in both control and MDMA subjects.
Personality Measures
Multidimensional Personality Questionnaire (MPQ). A 2 x 2 (drug condition x gender) multivariate ANCOVA with age as a covariate revealed a main effect of group on the control scale (F11,521 = 9.21; p = .004), with MDMA subjects scoring higher (indicating less impulsivity than controls. Post-hoc comparisons revealed that female MDMA subjects had higher control scores than all other experimental groups. A near sigrdficant effect of group was also observed on the harm avoidance scale (F[1,521 - 3.69; p = .060), with MDMA subjects reporting greater harm avoidance than controls. A main effect of gender was also observed on the harm avoidance scale (F11,521 = 5.03; p = .029), with posthoc testing indicating that female MDMA users had greater harm avoidance than all other experimental groups. Additionally, a gender effect was observed on the higher order scale of constraint (FJ 1, 52) = 5 . 0; p = .03), reflecting less impulsive, more conservative, and more harm avoidant personality traits. Post hoc testing revealed that female M`DM.A subjects had higher scores on this scale than all other subject groups. Group by gender interactions were observed on the alienation scale (F11,521 = 4.4; p - .004), the control scale (F11,52] = 6.4; p .01), the harm/avoidance scale (F[1,521 = 4.2; p .047), and the constraint scale (F[1,52] = 4 .3; p - .043).
Buss Durkee Hostility Inventory (BDH1). A 2 x 2 (group x gender) multivariate ANCOVA revealed a main effect of group on the indirect hostility scale, with M'DMA subjects reporting less hostility than controls (F[1,521 = 7.4; p = .009). Post-hoc testing revealed that both MDMA gToups reported significantly less indirect hostility than control females, but that neither NfDMA group was significantly different from male controls. A significant effect of gender was also observed on the indirect hostility scale (F11,52] = 6.7; p = .013), reflecting the previously mentioned scores in female MDMA users.
Eysenck Personality Questionnaire (EPQ). No drug or gender effects, or drug x gender interactions were observed on the sociability and impulsivity subscales of this questionnaire.
Correlations Between Biologic and Personality Measures
Correlation analyses between CSF 5-FEAA concentrations and personality scales were performed; analyses were restricted to scales in which significant g-roup or gender effects or group by gender interactions had been observed. Correlations were done for male and female subjects separately because of gender differences in CSF 5-1-11AA content.
MPQ. For female subjects, CSF -5-1-11AA was positively correlated with alienation (p<.0001), with near significant negative correlations on control (p = .06). For male subjects, there was a positive correlation between C5F 5-HIAA and harmlavoidance (p = .02), and a near significant positive correlation between CSF 5-HIAA and constraint (p = .07).
BDHI. Both female and male groups had near significant correlations between CSF 5-FI1AA and Indirect Hostility (p - .09 and p - A, respectively).
DISCUSSION
The major finding of the present study is that under controlled conditions, MDMA subjects have lower levels of CSF 5-111AA than control subjects matched for age, height, weight, gender, education, and other drug use. This finding, coupled with the observation that height and CSF 5-MAA are not negatively correlated in 1ADMA subjects, as they are in control subjects of this (see Results) and other (WodeHelgodt and Sedvall 1978; Stardey et al. 1985) studies, suggests that recreational MDMA use is associated with an alteration in central 5-HT metabolism. Although the nature of the MDMA-induced alteration in 5-HT metabolism is difficult to establish on the basis of CSF data alone, the fact that similar CSF 5-HIA.A decrements are evident in MDMAtreated monkeys with known serotonergic CNS deficits (Ricaurte et al. 1988c) suggests that CSF 5-HIAA reductions in MDMA users may reflect M13MA neurotoxicity.
The absence of a negative correlation between height and CSF 5-HIAA in MDMA subjects is noteworthy in one other respect. The inverse relation between height and CSF 5FRAA is thought to arise, at least in part, from a concentration gradient of 5-HIAA along the craniospinal axis (Sjostrom et al. 1975; Weir et al. 1973; Garelis et al. 1974). Its absence in MDMA users raises the possibility that MDMA perturbs those processes that normally contribute to the CSF 5-141AA gradient. One way that MDMA could produce this effect is by damaging ascending 5-HT axon projections to a greater extent than descending 5-HT projections. Indeed, recent findings in rodents (Molliver et al. 1990) as well as nonhurnan primates (Insel et al. 1989; Ricaurte et al. 1992) suggest that MDMA produces regioselective neurotoxic effects on central 5-HT systems, darnaging ascending 5-HT projections more than descending 5-HT projections.
Analysis of the CSF data also revealed that reductions in CSF 5-1-RAA were greater in women than in men (46% versus 20%). Although women could be more susceptible than men to MD1AA's 15-HT depleting effects (i. e--pharmacodynamic factors could be involved), the larger CSF 5-~ deficits in women are more likely to be exposure related, because women in the cohort weighed less than men yet generally reported taking the same dose (one tablet or capsule containing 100 to 150 mg). Moreover, review of the drug history data revealed that, on average, women had used MDMA more than men (115 times as compared to 85). These factors along with the fact that gender differences in susceptibility to MDMA neurotoxicity have not been noted in preclinical studies, suggest that pharmacokinetic rather than pharmacodynamic factors underlie the greater effect in women. In tl-ds regard, it is of note that MDMAexposed women, in addition to having reduced CSF 5-HIAA, also have reduced CSF HVA (Table 4), a finding that is in keeping with the observation that at high dosage, MDMA can damage dopaminergic as well as serotonergic neurons (Commins et al. 1987).
The prolactin response to L-tryptophan in MDMA users did not differ from that in controls. At first glance, this finding would appear to be at odds with that of a previous report (Price et al. 1988) indicating that recreational MDMA users might have blunted prolactin responses to L-tryptophan. However, as pointed out by the authors of that report, the decrements in prolactin response observed in that study cohort did not achieve statistical significance. Furthermore, recent findings in monkeys indicate that following MDMA injury, 5-HT axons innervating the hypothalamus (i.e., those Presumably subserving the prolactin response), unlike those projecting to the neocortex, recover in the months ensuing MDMA treatment.(Ricaurte et al, 1992). Given that on average, subjects in this study had last used MDMA 18 weeks prior to study participation, with some subjects abstaining from MDMA for as long as 2 years, it is possible that the lack of neuroendocrine findings is secondary to serotonergic axonal recovery at the level of those brain structures that -mediate the prolactin response to L-tryptophan. Alternatively, it is possible that central S-HT deficits that give rise to CSF 5-MAA deficits are insufficiently large or perhaps not appropriately located to give rise to a neuroendocrine functional deficit.
Differences between MDMA users and controls were found on measures of several personality traits thought to involve serotonin, including aggression, impulsivity, harm avoidance, and constraint. Further, female MDMA users, the MDMA subgroup with the greatest decrement in CSF 5-HIAA, were found to be the subgroup with the larger differences in personality. Somewhat unexpected was the direction of the personality differences, whkh indicated that MDMA users had decreased rather than increased impulsivity and hostility, as well as increased harm avoidance and constraint. Although this might seem surprising at first, the present results might be explained if one considers that the underlying basis for decreased CSF 5-MAA in MDMA users (presumably neurotoxic injury) may be different from that in patients groups exhibiting increased impulsivity and aggression (patients with personality disorders, violent offenders, suicidal individuals) - Specifically, it may be that the nature and regional distribution of central 5-HT deficits induced by MDMA are different from those in patients exhibiting impulsive and aggressive behaviors. Furthermore, in certain patient groups (e.g., patients with obsessive-compulsive disorder), drugs that promote net increases in serotonin activity have been found to decrease harm avoidant behaviors (e.g., compulsive rituals) (Chouinard 1992). Thus, there is precedent for decreased serotonin function being associated with increased constraint and harm avoidance. Moreover, a recent report indicates that humans with inherited monoandne oxidase A (MAO-A) deficiency, and therefore presumably an increase in brain serotonin, have increased, rather than decreased, impulsive aggressive behavior (Brunner et al. 1993). Finally, it should be noted that personality differences are not simply a confound of drug abuse tendencies, because substance abusers, unlike MDMA users in this study, typically have increased rather than decreased scores on measures of impulsivity (Fishbein et ad. 1989).
Several factors should be considered when interpreting these data. First, it is possible that the reduction in CSF 5-HIAA observed in MDMA users is related to a premorbid condition that antedated the use of MDMA. This possibility seems unlikely, however, because care was taken to screen out individuals with past or present psychiatric disorders in which 5-HT dysfunction has been implicated (including depression, anxiety, personality disorders, and alcoholism), and because no significant differences were found between controls and MDMA subjects on standardized psychiatric measures. Second, it could be argued that although MDMA was the drug of choice in most MDMA users, it was not the only drug used, and that other drugs are responsible for the observed changes. Because control subjects, like MDMA subjects, by design had exposure to other recreational drugs (albeit, to a lesser extent), and since few, if any, recreational drugs outside of the amphetamine class produce selective neurotoxic effects of the type produced by MDMA, this possibility also seems unlikely. Third, it could be that decreases in CSF 5-HIAA reflect a decrease in the synthesis, storage, release, or degradation of brain 5-HT, or an increase in the removal of 5-1-HAA from the CSF compartment that is unrelated to MDMA neurotoxicity. Although this is possible, as noted above, the fact that MDMA-treated monkeys with documented CNS 5-HT neurotoxicity (Insel et al. 1989; Ricaurte et al. 1988c) show similar CSF 5-HLA-A deficits to those here documented in MDMA users mitigates against this possibility.
With regard to the finding that MDMA users have lower scores on scales of impulsivity and control, it is also important to consider the possibility that these differences were secondary to factors unrelated to MDMA use. For example, it is possible that MDMA sub~jects, who were often strong advocates for its beneficial effects, were biased in their responses on personality questionnaires in order to highlight positive aspects of their drug experience. Although possible, it would be difficult to explain why MDMA users independently and selectively chose to respond in a biased Manner on scales relating to impulsivity and hostility (since similar "positive" answers could be given on scales reflecting confidence, positive affect, alienation, and social potency). The negative correlation between CSF 5-11IAA and measures of control would also be somewhat curious if personality differences were due to subject bias, given that subjects were not privy to CSF monoaTnine levels at the time questionnaires were completed. An alternate explanation for personality differences is that "control" subjects were unusually impulsive, and that differences in M`DN1A subjects are actually a reflection of abnormalities in the control group. This possibility seems less than likely however, when one considers that impulsivity scores in the control group fall into the range previously reported in "normal" populations.
Predictions regarding the amount of MDMA required to induce 5-HT neural damage in humans are difficult to make based on the present study, because the dose and duration of NOMA use differed among subjects, and because estimates of MDMA exposure are based on self-reports. Additional studies are needed to establish whether individuals who have taken smaller amounts of MDMA also show evidence of altered 5-HT metabolism and to determine why women are more affected than men. Controlled clinical studies are also indicated to ascertain whether dexfenfluramine, a cbnically prescribed appetite suppressant, produces sin-Lilar changes, since dexfenfluramine is taken by more people, and more frequently than MDMA, and is highly toxic to 15-HT neurons in nonhuman primates (Ricaurte et ad. 1991).
In summary, the present results indicate that MDMA neurotoxicity, heretofore only documented in animals, may generalize to humans. When evaluated under controlled conditions, recreational MD&LA users have reduced CSF 51HLAA levels compared to matched controls. In addition, when compared to controls, MDMA subjects are less impulsive, more harm-avoidant, and have decreased indirect hostility, supporting the notion that these personality characteristics are modulated by serotonin. Further studies are needed to confirm and extend these observations. In particular, specific pharmacologic or physiologic challenge of central 5-HT systems in MDMA-exposed individuals may provide insight into the role of 5-HT in normal human brain function and may shed light on the involvement of 5-HT in neuropsychiatric disease states.
ACKNOWLEDGMENTS
This study was supported by a U.S. Public Health Service Research Grant (ROlDA05938) from the National Institute on Drug Abuse, and General Clinical Research Grant (GCRC)
References
Anon (1992): Drug Culture. Lancet 339:117
Battaglia G, Yeh SY, OHearn E, Molliver ME, Kuhar MJ, DeSouza EB (1987): 3,4-Methylenedioxy-methamphetamine and 3,4methylenedioxyamphetaniine destroy 5-HT tern,dnals in rat brain: quantification of neurodegeneration by measurement of [3H]paroxetinelabeled 5-HT uptake sites. J Pharmacol Exp Ther 242:911-916
Ben Menachern E, Persson L, Schecter PJ (1989): Cerebrospinal fluid parameters in healthy volunteers during serial lumbar punctures. J Neurochern 52:632-635
Bowers MB, Gerbode FA (1968): Relationship of monoamine metabolites in cerebrospinal fluid to age- Nature 219: 1256-1257
Brewerton TD, Berrettini WH, Numberger JI (1987): Analysis of seasonal fluctuations of CSF monoamine metabolites and neuropeptides in normal controls: findings with 5-HIAA and HVA. Psychiatry Res 23:257-265
Brunner HG Nelen M, Breakefield XO, Ropers; HH, van Oost BA (I~W3): Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 262:578-580
Buss AH, Durkee A (1957): An inventory for assetsing different kinds of hostility. J Consulting Psychol-21:343-349
Chouinard G (1992): Sertraline in the treatment of obsessive compulsive disorder: Two double-blind placebo-controlled studies. Int Clin Psychopharmacol Suppl 2:37-41
Coccaro EF (1992): Impulsive afression and central serotonergic system function in humans: an example of a dimensional brain-behavior relationship. Int Clin Psychopharmacol 7:3-12
Commins DL, Vosmer G, Virus R, Woolverton W, Sciden L (1987): Biochemical and histological evidence that rnethylenedioxymethylamphet~e (MDMA) is toxic to neurons in the rat brain. J Pharmacol Exp Ther 241:338345
Eisner B (1989): Ecstasy: The MDMA Story. Berkeley, CA, Ronin Publishing Inc
Eysenck HJ (1976): The Measurement of Personality. Baltimore, University Park Press
Fishbein DH, Lozovsky D, Jaff e JH (1989): Impulsivity, a~gression and neuroendocrine responses to serotonergic stimulation in substance abusers. Biol Psychiatry 25:10491066
Carelis E, Young S, Lal S, Sourkes T (1974): Monoamine metabolites in lumbar CSF: The question of their origin in relation to clinical studies. Brain Res 79:1-8
Gottfries C, Gottfries 1, Johansson B, Olsson R, Persson T, Roos B, Sjostrorn R (1971) : Acid monoamine metabolites in human cerebrospinal fluid and their relation to age and sex. Neuropharmacology 10:665-672
Henry JA Jeffreys KJ Dawling S 1992 Toxicity and deaths from 3,4 methylenedioxymetamphetamine ("ecstasy") Lancet 340 384-387
Hildbrand J, Bourgeois F Buyse M (1990) Reproducibility of monoamine metabolite measurements in human cerebrospinal fluid Acta Neurol Scand 81 427-430
Insel TR Battglia G, Johannsen JN, De Souza EB (1989) 3,4 methylenedioxymetamphetamine (MDMA "ecstasy") selectively destroys brain 5-HT terminals in rhesus monkey. J Pharmacol Exp Ther 249-.713-720
Kilpatrick I, Jones M, Phillipson 0 (1986): A semiautomated analysis method for catecholan-dnes, indolearnines, and some prominent metabolites in microdissected regions of the nervous system: An isocratic HPLC technique employing coulometric detection and minimal sample preparation. J Neurochern 46:1865-1876
Linnoila M, Virkkunen M, Scheinin M, Nautila A, Rimon R, Goodwin FK (1983): Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from non-impulsive violent behavior. Life Sci 33:26092614.
Lints CE, Harvey ]A (1969): Altered sensitivity to footshock and decreased content of 5-HT following brain lesions in the rat. J Comp Physiol Psychol 67:23-39
Messing RB, Lytle LD (1977): Serotonin-containing neurons: Their possible role in pain and analgesia. Pain 4:1-21
Molliver ME, Berger LTV, Mamounas LA, Molliver DC, O'Hearn EG, and Wilson MA (1990): Neurotoxicity of MDMA and related compounds: Anaton-dc studies. Ann NY Acad Sci 600:640-664
Nencini P, Woolverton W, Seiden L (1988): Enhancement of morphine-induced analgesia after repeated injections of rnethY]enedioxymethamphetan-tine. Brain Res 457.136142
O'Heam EG, Battaglia G, De Souza EB, Kuhar MJ, Molliver ME (1988): Methylenedioxyamphetan-Line (MDA) and methylenedioxymethamphetamine (MDMA) cause ablation of serotonergic terminals in forebrain: Immunocytochemical evidence. J Neurosci 8:2788-2803
Peroutka SJ (1987): Incidence of recreational use of 3,4methylenedioxymethamphetamine (MDMA, "Ecstasy") on an undergraduate campus. N Engl J Med 317 (24): 1542-1543
Peroutka SJ, Pascoe N, Faull KF (1987): Monoamine metabolites in the cerebrospinal fluid of recreational users of 3,4methylenedioxymethamphetamine (MDMA;'Tcstasy"). Res Commun Substance Abuse 8:125-138
Post RM, Ballenger JC, Goodwin FK (1980): Cerebrospinal fluid studies of neurotransn-titter function in manic and depressive illness. In Wood J14 (ed), Neurobiology of Cerebrospinal Fluid: New York, Plenum Press, 685-717
Price LH, Charney DS, Delgado PL, Goodman WK, Krystal JH, Woods SW, Heninger GR (1990): Clinical studies of HT function Using I.V. L-tryptophan. Prog Neuropsychopharmacol Biol Psychiatry 14:459-472
Price LH, Ricaurte GA, Krystal JH, Heninger GR (1988): Neuroendocrine and mood responses to intravenous L-tryptophan in 3,4methylenedioxymethamphetamine (MDMA) users. Arch Gen Psychiatry 46:20~22
Randall T (1992): Ecstasy-fueled "rave" parties become dances of death for English youths. JAMA 269:1505-1506
Ricaurte GA Forno Wilson DeLanney, Irwin Molhver ME, Langston JW (1988a): (±)3,4-Methylenedioxymethamphetan-dne (NMMA) selectively damages central serotonergic neurons in non-human primates. JAMA 260:51-55.
Ricaurte GA, DeLanney LE, Irwin 1, Langston JW (1988b):
Ricaurte GA, DeLanney LE, Wiener SG, Irwin I, Langston JW (1988c): 5-hydroxyindoleacetic acid in cerebrospinal fluid reflects serotonergic damage induced by 3,4-methylenedioxymethamphetamine in CNS of non-human pnmates. Brain Res 474:359-363
Ricaurte GA, Finnegan KT, Irwin 1, Langston JW (1990): Aminergic metabolites in cerebrospinal fluid of humans previously exposed to MDMA: Preliminary observations. Ann NY Acad Sci 600:699-710
Ricauxte GA, Molliver ?~E, Martello MB, Katz JL, Wilson MA, Martello AL (1991): Dexfenflurarnine neurotoxicity in brains of non-human primates. Lancet 338:1487-1488
Ricaurte GA, Martello A, Katz JL, Martello MB (1992): Lasting effects of (±)3,4-methylenedioxymethamphetamine on central serotonergic neurons in non-human primates. J Pharmacol Exp Ther 261(2):616-622
Richardson P (1992): Organization of 5-HT neurones regulating central pain. In Bradley P, Handley S, Cooper S, Key B, Barnes N, Coote J (eds), 5-HT, CNS Receptors and Brain Function. Oxford, Pergamon Press, pp. 335-347
Roy A, Adinoff B, Linnoila M (1988): Acting out hostility in normal volunteers: negative correlation with levels of 5-HLkA in cerebrospinal fluid. Psychiatry Res 24:187-194
Schmidt CJ (1987). Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J Pharmacol Exp Ther 240:1-7
Sjostrom R, Eckstedt J, Anggard E (1975). Concentration gradients of monoamine metabolites in human cerebrospinal fluid. J Neurol Neurosurg Psychiatry 38:666-668
Smith GM, Egbert LD, Markowitz RA, Mosteller F, Beecher HK (1966): An experimental pain method sensitive to morphine in man: The submaximum effort tourniquet technique. J Pharmacol Exp Ther 154:324-332
Spitzer RL, Williams JBW (1982): Structured clinical interview for DSM-11I-R. New York, New York State Psychiatric Institute, Biometrics Research
Stardey M, Traksman-Bendz L, Dorovinj-Zis K (19W): Correlations between aminergic metabolites simultaneously obtained from human CSF and brain. Life Sci 37.12791286
Stone DM, Stahl DS, Hanson GL, Gibb JW (1986): The effects of 3,4-methylenedioxymethamphetan-dne (MDMA) and 3,4methylenedioxyamphetamine on monoaminergic systems in the rat brain. Eur J Pharmacol 128:41-48
Tellegen A (1982): Multidimensional Personality Questionnaire. Copyright by Auke Tellegen
Weir RL, Chase TN, Ng LK, Kopin IJ (1973): 5-Hydroxyindoleacetic acid in spinal fluid: relative contribution from brain and spinal cord. Brain Res 52:409-412
Sharpless N (1986): Detemiination of total 3-methoxy-4-hy- Wode-Helgodt B, Sedvall C (1978): Correlations between droxypheny1glycol in plasma using reversed-phase liq- height of subject and concentrations of monoamine meuid chromatography with electrochen-ical detection. J tabolites in cerebrospinal fluid from psychotic men and Chromatography 377:101-109 women. Commun Psychopharmacol 2:177-183
Last Updated (Monday, 20 December 2010 19:29)












