Neurochemical and Neurohistological Alterations in the Rat and Monkey Produced by Orally Administered Methylenedioxymethamphetamine (MDMA
Drug Abuse
Neurochemical and Neurohistological Alterations in the Rat and Monkey Produced by Orally Administered Methylenedioxymethamphetamine (MDMA)'
WILLIAM SLIKKER, JR.,*'§ SYED F. ALI,**t ANDREW C. SCALLET,*'§ CHARLES H. FRITH,t,§ GLENN D. NEWPORT,* AND J. R. BAILEY*
*Pharmacodynamics Branch, Division qfReproductive and Developmental Toxicology, National Centerfor Toxicological Research. Jefferson, Arkansas 72079, and tDepartment of Biochemistry and Molecular Biology, Departmeni ofPathology, and §Department ofPharmacology and Interdisciplinary Toxicology, University of Arkansas for Medical Sciences, Little Rock, Arkansas 72205
Neurochemical and Neurohistological Alterations in the Rat and Monkey Produced by Orally Administered Methylenedioxymethamphetamine (MDMA). SLIKKER, W., JR., Au, S. F., ScALLET, A. C., FRITH, C. H., NEWPORT, G. D., AND BAILEY, J. R. Toxicol Appl. Pharmacol. 94, 448457. MDMA is an amphetamine analog prescribed by some health professionals in the field of psychotherapy and used as a recreational drug by the general public. In recent reports, investigators have suggested that MDMA produces acute neurotoxicity when administered by subcutaneous injection. In order to determine if MDMA produces lasting neurochemical alterations after oral administration, groups of six rats (adult male Sprague-Dawley) were dosed by gavage With either 40 or 80 mg/kg of MDMA or saline vehicle once every 12 hr for 4 days. These rats were terminated 2 weeks after the first dose along with an additional group of rats (80 mg/kg) terminated 4 weeks after the first dose. Brain regions including the hippocampus (H), caudate nucleus (CN), hypothalamus (HY), frontal cortex (FC), and brain stem (BS) were analyzed by HPLC with electrochemical detection for concentrations of dopamine (DA), dihydroxyphenytacetic acid (DOPAC), homovanillic acid (HVA), serotonin (5-HT), 5-hydroxyindoleacetic acid (5HIAA), Ad norepinephrine (NE). In the CN, 40 mg/kg MDMA produced no change in DA, DOP41- or HVA, but a 5060% decrease in 5-HT and 5-HIAA concentrations was observed at 2 weeks. Similar effects were observed at 80 mg/kg at both 2 weeks and 4 weeks. A temporary decrease was also seen in DA (2 1 %) and in HVA (34%) 2 weeks but not 4 weeks after the 80 mg/ kg dose regimen. In the H. MDMA (40 or 80 mg/kg) produced no change in NE, but a 50-60% decrease was seen in 5-HT and 5-HLkA concentrations at 2 weeks. Concentrations of 5-HT and 5-HIAA were significantly decreased in the HY and FC by all MDMA treatments, but DA and DOPAC concentrations were not altered as compared to vehicle controls. BS was least affected by treatment with no change in DA, DOPAC, or 5-HIAA concentrations and only a slight decrease in 5-HT (19-33%) concentrations at 2 weeks but not at 4 weeks. To determine the sensitivity of the nonhuman primate to MDMA, a total of nine rhesus monkeys were dosed with vehicle or 5 or 10 mg/kg MDMA (n = 3 ) by gastric intubation twice per day for 4 days. One month after MDMA dosing, a dose-related reduction from vehicle control values for 5-HT and 5-HIAA was observed. These results indicate that the monkey may be more sensitive than the rat to the persistent sercitonergic neurotoxicity of MDMA. Histological assessment in the rat with a stain specific for degenerating fibers and terminals 18 hr after a single treatment with MDMA (40 and 80 mg/ kg, po) indicated a significant enhancement of the density of silver-impregnated terminals in the CN as compared to vehicle controls. Selective reductions in brain 5-HT and 5-HIAA concentrations, along with histologically observed neuronal degeneration, suggest that orally administered MDMA produces selective destruction of serotonergic nerve terminals. c iiiss Academic Press, Inc.
Methylenedioxymethamphetamine (MDMA) is an amphetamine analog and has been used by some health professionals in the field of psychotherapy and by the general public as a recreational drug (Greer, 1985; Frith et al., 1987). Psychotherapists suggest that the drug enhances communication between therapist and patient by apparently imparting a profound sense of peacefulness and interpersonal trust to the user. MDMA is known as "Ecstasy" on the street and users testi~v that it intensifies emotional feelings without distorting the senses. MDMA has recently been reported to be a potential neurotoxicant in the rat after subcutaneous administration (Gibb et al., 1986; Schmidt, 1987). MDMA decreased tryptophan hydroxylase (the ratelimiting enzyme for serotonin synthesis) but not tyrosine hydroxylase activity in the hippocampus, frontal cortex, and striaturn of the rat (Gibb et al., 1986). MDMA also reduced the content of 5-HT itself as well as its metabolite (5-HIAA) in several brain regions in a biphasic manner; an initial early decrement returned to control values by 24 hr while a subsequent decrease lasted at least 7 days (Schmidt, 1987). Neuropathological studies have recently been rep4rted for MDMA (Commins et al., 1986) and a closely related structural analog, methylenedioxyamphetamine, MDA, (Riqaurte el al.,1985) after subcutaneous administration.
The use of findings of MDMA-induced neurotoxicity in the rat to predict human risk is difficult. The parenteral route of MDMA administration described in previous studies (Gibbet al., 1986; Schmidt, 1987; Commins et al., 1987) is one source of uncertainty since MDMA is usually administered orally in humans (Greer, 1985; Shulgin and Nichols, 1978) and MDMA and its congeners undergo hepatic metabolism (Glennon, 1984; Bradyel al., 1986; Gollamudi et al., 1988). Another source of uncertainty involves extrapolation of the finding in the rodent to the human population. With the knowledge that certain neurotoxicants, such as MPTP, are much more potent in the human and nonhuman primate than the rodent (DAmato et al., 1985), it became warranted to investigate the sensitivity of the monkey to, the effects of MDMA after oral administration.
The present study was designed to determine if the decrease in 5-HT and metabolite concentration observed after subcutaneous administration of MDMA occurs after oral administration. The time course and selectivity of the MDMA-induced alteirations as well as associated neurodegeneration as quantified by silver staining were also assessed. Finally, the effects of orally administered MDMA in the monkey were compared to those observed in the rat.
MATERIALS AND METHODS
Chemical. MDMA was kindly supplied by Dr. David Nichols, Department of Medicinal Chemistry, Purdue University, and National Institute on Drug Abuse, Washington, D.C. The structure was confirmed by chemical ionization mass spectrometry at NCTR. The purity and stability of the MDMA (>99%) has been previously described (Frith ef al., 1987). Other chemicals and solvents used were HPLC grade.
Experimental design (rodent studies). Adult male SpragucDawicy rats (Charles River, PonW, MI) were used in the studies. Animal quarantine, caging, and feeding provisions have been previously described (Frith et al., 1987). A total of 24 rats (four groups of n - 6) were terminated for neurochernical evaluation on the same experimental day in balanced alternation between groups in order to control for any effects of circadian variation. Based on the data of Ricaurte el al. (1985) a regimen of MDMA treatment (eight repeated oral doses of vehicle (saline) or 40 or 80 mg/kg once every 12 hr for 4 days at 2 ml/kg) was employed to evaluate the presence, doserelatedness, and persistence of neurochernical alterations following repeated MDMA administration.
Experimental design (monkey studies). Adult female rhesus monkeys ranging in weight from 4.2 to 9.5 kg and in age from 8 to 15 years were maintained as previously described (Slikker ei al., 1983). A total of nine monkeys (three per group) were dosed with vehicle (water) or 5 or 10 mg/kg MDMA by gastric intubation twice per day (9 Am and 4 Pm) for 4 days. Monkey body weights were recorded the week before dosing and 3 days after the last dose. One month after the last MDMA dose, the monkeys were overdosed with pentobarbital and their brains removed, quickly dissected into different regions, and frozen on dry ice and ad~yzed for 5-HT and 5-HIAA concentrations as described below.
FiG. 1 A. Darkfield photomicrograph of a section through the caudate nucleus of a control rat Note the absence of any silverimpregnated axons which would appear as white fibers due to scattered fight from the silver deposits contained in the axons as in Fig. 1 B. Figs. IA and 1 B: M indicates patches of myelinated fibers characteristic of the caudate nucleus. Small arrows indicate silverimpregnated fibers, whereas large arrows indicate intrinsic neurons of the caudate nucleus. Both micrographs X420.
Brain dissection. Six rats, from each group, at each time point were randomly selected and killed by dccapitation, and their bi-Ains were removed quickly and dissected midsagittally into two halves. Each half was dissected into frontal cortex (FC), caudate nucleus (CN), hippocampus (H), hypothalamus (HY), frontal cortex (FC), and brain stem (BS) following the guidelines of Glowinski and Iverson (1966); tissues were placed on dry ice and stored at -70*C until processed.
The monkey brains were dissected on an icc-cold plate according to a minor modification of the method of Brown et aL (1979) using the atlas of Szebenyi (1970). The cortex was removed as four large pieces: The prefrontal cortex, the premotor/precentral gyrus cortex, the preoptic/parietal cortex, and the occipital cortex. Approximately 600 mg of tissue of the ventral frontal cortex was assayedL The basal ganglia, thalamus, hypothalamus, and brain stem were removed as described by Brown et aL (1979), except the basal ganglia was further dissected into the caudate nucleus and putamen and the hippocampi were rolled out from the surrounding temporal gyrus and frozen as two entire, separate pieces, one from each hemisphere. Approximately 450 mg of the ventral caudate nucleus, 600 mg of the rostral brain stem, and the entire right hippocampus were assayed. As each sample was removed, it was immediately frozen on dry ice and each dissection was completed within 5 min of removal of the brain from the animal.
Neurotransmitter and metabolfte assay. Neurotransmitters and their acid metabolites were resolved by highperformance liquid chromatography (HPLQ and quantified by electrochemical detection (Ali ei al., 1986). Briefly, each individual region of the brain was weighed and placed in a measured volume (10: 1. v/w) of 0.2 N perchloric acid containing 250 ng/ml of the internal standard 3,4dihydroxbenzylamine (DHBA). Brain tissue was then disrupted by ultrasonication and centrifuged (1000g. 5 min), and 150 jAI of the supernatant was removed and filtered through a 0.2-iAm microfilter (MFI microcentrifuge filter, Bioanalytic Systern (BAS), W. Lafayette, IN). Aliquots of 25 jul representing 10 mg of brain tissue were injected directly onto the HPLC system for separation of the neurotransmitters and their metabolites.
Neuropathology. In order to determine the presence of neuronal toxicity or death and its neurochemical basis, 11 rats were terminated for silver impregnation of degenerating neuronal elements. The caudate nucleus was selected for analysis bemuse theleurochemical data indicated a 50% reduction of 5-HT concentration in this brain region after MDMA treatment and earlier studies by Ricaurte et al. (1985) demonstrated the caudate sensitive to neurodegeneration by a MDMA congener. Four groups were perfused on a single experimental day. One group (n = 3) was terminated 18 hr after a single 80 mgl kg dose of MDMA and another group was terminated 18 hr after 40 mglkg in order to evaluate the earliest signs of dose-related toxicity from our MIDMA regimen. Two control rats were intubated with an equal volume of saline 18 hr prior to termination. An additional group of three animals were terminated at 2 weeks following the eightdose regimen in order to determine the correspondence of histological alterations to the neurochemistry results.
FIG. 1 B. Similar section through the caudate of an animal treated 2 weeks previously with 80 m&lkg po of MDMA. Small arrows indicate several small fibers presumed to be degenerating argyrophilic axons
Rats were anesthetized with Nembutal (Abbott Laboratories) and perfused through the left ventricle of the heart with a needle clamped into the ascending aorta. An initial flush of 20-30 m] of 0.9% saline buffered to pH 7 with 0.1 m potassium phosphate was followed by 100200 m] of pH 7 phosphate-buffered 10% formalin. The whole brains were removed and stored overnight in the same buffered formalin. On the following day, all brains were blocked and a series of 30 pm sections cut with a vibratome (Lancer Instruments Co.) through the caudate/putamen of each rat at the level of brcgma (Paxinos and Watson, 1982). Two nonadjacent sections were stained with cresyl violet for neuronal Niss] substance, and according to a modification of the silver impregnation method of Finkand Heimer(1967) fordegcnerating fibers and cell bodies (Nadler and Evenson, 1983). To confirm that the method was sensitive to and specific for degenerating neuronal elements, sections from rats treated I week previously with 9 mg/kg of the hippocampal neurotoxin trimethyltin (Paule el aL, 1986) were included with the MDMA sections in each batch that was processed for silver degeneration.
Silver-impregnated terminals could be observed in the caudate sections as spidery, multiprocess varicose fibers generally found in proximity to cell bodies (Figs. I A and I B). Quantification of the silverimpregnated terminals in the caudate regions was performed by an observer blind to the group identification of the sections. Four randomly chosen, non-overlapping 20-mm-diameter fieldsof-view were examined at a magnification of 40OX from each of the two caudate sections stained per brain. The total number of terminals observed in all fields was then divided by 0.0157 MM2 (the total area of 30-jurnthick tissue comprising the eight fields counted) in order to derive an estimate of the density of stained terminals in each MDMA or control rat.
Siatistical analysis. Main effects of MDMA treatment on both neurochemistry and neuropathology were determined by one-way analyses ofvariancc. The null hypothesis that the treated and control groups had the same mean was rejected when F ratios exceeded the 95% conHence levels. Subsequent individual comparisons betwecn the groups %ith a p < 0.05 by the Fisher's least significant difference approach (Winer, 197 1) were considered statistically reliable.
RESULTS
Neurochemistry (rodent studies). In the CN, 40 mg/kg MDMA produced no change in DA, DOPAC, or HVA concentrations but reductions in 5-HT (51%, p < 0.01) and 5HIAA (63%, p < 0.01) concentrations were observed 2 weeks after the first dose (p < 0.0 1). Similar effects (48-58% decreases, p < 0.0 1 from vehicle control) were observed at 80 mglkg both at 2 and 4 weeks after the first dose. A transient decrease from control levels was also seen in DA (21%, p < 0.05) and HVA (34%, p < 0.05) 2 weeks but not 4 weeks after the 80 mglkg dose regimen. DA and HVA were not altered by the 40 mg/kg treatments (Table 1). 4
In H, MDMA (40 mglkg) induced no change in NE (data not shown) but 5-HT and 5-HIAA, concentrations were about equally decreased (5558%) 2 weeks after the first dose (p < 0.01). Similar effects (51-60% decrease, p values < 0.01 from vehicle) were seen at 2 and 4 weeks after the 80 mg/kg doses (Table 2).
In HY and FC, DA and DOPAC concentrations were unchanged by treatment (data not shown) whereas 5-HT and 5-HIA.A exhibited a 23-60% reduction in concentration with treatment (p values < 0.05; Table 2).
The BS was least affected by MDMA treatment with no changes in DA, DOPAC, or 5HIAA concentrations and reductions of only 23 and 19% in 5-HT concentrations 2 weeks after the 40 or 80 mglkg doses, respectively (p values < 0.05, Table 2). At four weeks, although the 80 mglkg dosed rats had lower BS concentrations of 5-HT than those of controls, the differences were not statistically significant.
Neurochemistry (monkey studies). The four brain areas examined in the monkey all tended to show a dose-related decrease in 5 HT and 5-HIAA concentrations as the MDMA dose was increased from 0 to 10 mg/kg po (Table 3). Both the FC and H concentrations of 5-HT exhibited a 70-80% reduction from control values after the 10 mg/kg po dose and the H values were significantly different at the p < 0.0 1 level. Serotonin and 5-HIAA content of the H and FC was less
markedly affected but still significantly ' reduced by the lower 5 mglkg dose as well (p < 0.05). Smaller MDMA-induced reductions in CN 5-HT and 5-HIAA concentrations (45%) did not achieve statistical significance. As in the rat, MDMA produced lesser reductions in 5-HT and 5-HIAA concentrations inthe brain stem which were not significantly different from controls. No significant body weight alterations occurred during or within 3 days after dosing.
Neuropathology. Positive control (trimethyltin treated) sections included in the batch processed for silver staining showed numerous large degenerating fibers and tenninals in the hippocampus as well as degenerating cell bodies in layer 5 of cerebral cortex (not shown). Quantitation of degenerating terminals in MDMA rats revealed a 90% increase in the density of affected terminals 18 hr after a single 40 mg/kg dose (p < 0.05) and a 105% increase after 80 mg/kg, (p < 0.0 1), (see Fig. 2). An additional group of rats that had been treated 2 weeks before termination with four doses of 80 mglkg MDMA also exhibited a significantly enhanced number of silverstained terminal processes in the CN (p < 0.05, see Figs. 1 and 2).
DISCUSSION
These data demonstrate that MDMA induces neurochemical alterations in rats after oral administration, the most common route of administration in the human. The effects of MDMA were quite marked (55% reduction in frontal cortex 5-HT concentration) aslong as 4 weeks after treatment. This indicates that MDMA effects on the serotonergic system are not transient but long-lasting. Recent preliminary studies from our laboratory (Ali et al., 1987) confirm the long-lasting effects of MDN4A in that a significant reduction of 5-HT concentration was observed in the hippocampus of female rats 4 months after eight successive oral doses of MDMA (40 mg/kg). In contrast, MDMA produced only transient (2 weeks) alterations of the DA and NE systems which were no different from control levels by 4 weeks. This transient effect of MDMA on the catecholaminergic system is consistent with the lack of effect of MDMA on tyrosine hydroxylase (Gibb et al., 1986) and dopamine receptors (Ali et al., 1986; Scallet et al., 1988).
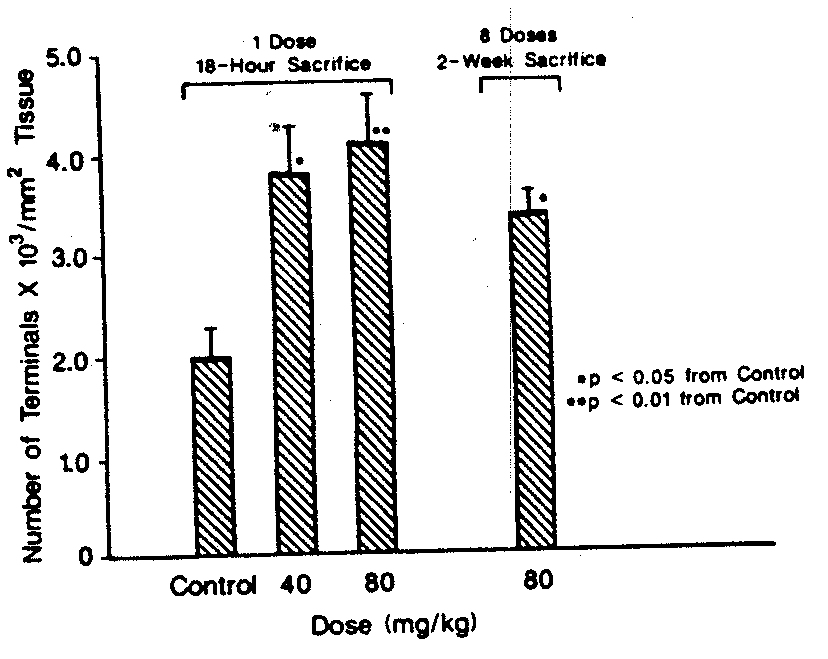
FIG. 2. The means ± SE are shown for the density of silver-impregnated terminals in caudate nucleus sections counted by an observer unaware of treatment identifications. Two sections per rat from two to three rats per treatment group were scored as described in the text. All MDMA-treated groups had significantly more stained terminals per square millimeter of tissue area than controls (*p < 0.05, **P < 0.0 1).
In the monkey, oral doses of MDMA (5 or 10 mg/kg) resulted in a dramatic dose-related decrease (7183% reduction) in 5-HT concentration in the frontal cortex and hippocampus. As in the rat, the brain stem was least affected, exhibiting only a 25% reduction of 5-HT as compared to vehicle control values.
These lasting changes were observed I month after MDMA dosing and occurred in the absence of body weight loss. The regional effects of MDMA on the serotonergic system are summarized in Table 4 for both the rat and monkey. It is interesting to note that the greatest losses of serotonin content occurred in those brain areas containing only serotonergic axons and nerve terminals (Cooper et aL, 1984). Those areas supporting serotonergic. cell bodies (brainstem) were less severely affected by MDMA treatment. These neurochernical data suggest that the effects of MDMA on the serotonergic system are predominantly directed toward the terminals rather than the soma of the neuron.
The increase in the density of silver-staining caudate axons of MDMA rats compared to vehicle controls also supports the concept of a nerve terminal site of M13MA toxicity.
These neuropathological signs of terminal de generation were observed as early as 18 hr after MDMA administration and lasted for at least 2 weeks thereafter. These findings with MDMA are consistent with those reported by Ricaurte et a[ (1985) for NIDA, a structurally related amphetamine derivative, and raise concern about the neurotoxic potential of this chemical class of psychedelic stimulants in humans. In the rats studied here, the tryptophan or monoamine oxidase pretreatments typically used to enhance serotonergic immunohistochemical staining (Cooper et aL, 1984) were avoided because of their potential interaction with the MDMA treatment of interest. However, in a separate set of tryptophan pretreated rats studied 4 months following treatment (Scallet ez aL, 1988), the dorsal rapheneurons visualized with an anti-serotonin antisera were not obviously different in numeric density between MDMA and control rats. Although these findings further support a nerveterminal site of action for MDMA, direct studies of the dorsal rapheneuron response to acute MDMA treatment will be required to establish whether actual death of certain brain stem cell-bodies might occur in response to this serotonergic toxicant.
TABLE 4
The concern over the route of MDMA administration is heightened because Molliver et aL(1986) reported that direct intracerebral injection of MDMA did not effect the appearance of axons revealed by a specific stain for their content of serotonin. These data suggest that a peripheral metabolite of MDMA may be responsible for the neurotoxicity. Our rodent data clearly indicate the profound effects of MDMA on the serotonergic system (over 50% depletion of 5-HT and 5-HLkA) in selected brain regions after oral administration.
The repeated doses of MDMA (40 and 80 mg/kg) used in these rodent studies are approximately 15 to 30 times the usual human oral MDMA dose (Shulgin and Nichols, 1978) although oral doses of MDMA of 50 and 100 mg/kg administered to rats once daily for 28 days have been reported to produce reduced weight gain and minor alteration of clinical chemistries but no histopathologic changes or deaths (Frith et at., 1987).
The significant effect of orally administered MDMA at doses as low as 5 mg/kg in the monkey suggests that the nonhuman primate is more sensitive than the rodent. At I month after MDMA treatment, the percentage of frontal cortex and hippocampal 5-HT depletion in the monkey was greater than that observed in the rat I month after 80 mg/ kg MDMA or 2 weeks after 40 mg/kg MDMA administered under the same regimen (Table 4). These data enhance the concern for human risk since the 5 mg/kg oral MDMA dose is approximately twice the usual human dose (Shulgin and Nichols, 1978) albeit eight such doses were administered over a 4-day period in the case of the monkey. Although the lowest effective oral dose of MDMA is yet to be determined, the present data in the rat and especially in the monkey clearly indicate that orally administered MDMA is an effective serotonin depleter and potential neurotoxicant at doses which do not produce morbidity and are relevant to human exposure.
ACKNOWLEDGMENTS
We thank Mr. George Lipe and Ed Wood for their technical assistance and Ms. Barbara Jacks for preparing the manuscript.
REFERENCES
ALI, S. F., SLIKKER, W., JR., SCALLET, A. C., AND FRITH, C. H. (1987). Neurochemical and neuropathological changes produced by methylenedioxymethamphetamine (MDMA) in different regions of rat brain. J. Neurochem. 48, S 112.
ALI, S. F., SLIKKER, W_ JR., NEWPORT, G. D., AND GOAD, P. T. ( 1986). Cholinergic and dopaminergic alterations in the mouse central nervous system following acute trimethyltin exposure. Acia Pharmacol. Tox icol. 59, 179-188.
BRADY, J. F_ Di STEFANo, E. W, AND CHO, A. K. (1986). Spectral and inhibitory interactions of (±)-3,4methylenedioxymethamphetamint (MDMA) with rat hepatic micro-omes. Life Sci. 39, 1457-1464.
BROWN, R. M_ CRANE, A. M_ AND GOLDMAN, P. S. (1979). Regional distribution of monoamines in the cerebral cortex and subcortical structures of the rhesus monkey: Concentration and in vivo synthesis rates. Brain Res. 168, 133150.
COMMINs, D. L., VOSMER, G., VIRUS, R. M., WOOL, VERTON, W. L., SCHUSTER, C. R., AND SEIDEN, L. S. (1987). Biochemical and histological evidence that methylenedioxymethylamphetamine (MDMA) is toxic to neurons in the rat brain. J. Pharmacol. Exp. Ther. 241, 338-345.
COOPER, J. R., BL00m, E. E., AND ROTH, R. H. (1984). The Biochemical Basis of Neuropharmacology, 4th ed., pp. 233-235. Oxford Univ. Press, New York.
D'AMATo, R., LiPMAN, Z. R, AND SNYDER, S. H. (1985). Selectivity of the Parkinsonian Neurotoxin MPTP: Toxic metabolite MPP* binds to neuromelanin. Science 231, 987~989.
FINK, R. R, AND HEIMER, L. (1967). Two methods for selective silver impregnation of degenerating axons and their synaptic endings in the central nervous system. Brain Res. 4, 369-374.
FRITH, C. H_ CHANG, L. W., LAMN, D. L_ WALLS, R. C., HAMM, J., AND DOBLIN, R. (1987). Toxicity of methylenedioxymethamphetamine (MDMA) in the dog and the rat. Fundam. Appl. Toxicol. 9,110-119.
GIBB, J. W., HANSON, G. R., AND JOHNSON, M. (1986). Effects of (+)-3,-4methylenedioxymethamphetamine [(+)MDMAJ and (-~3,4methylenedioxymetharnphetamine [(-)MDMAJ on brain dopamine serotonin and their biosynthetic enzymes. Neuroscience 12, 608.
GLENNON, R. A. (1984). Hallucinogenic phenylisopropylamines stereochemical aspects. In CRC Handbook ofStereoisorners: Drugs in Psychopharmacology (D. F. Smith, Ed.), pp. 327-368. CRC Press, Boca Raton, FL.
GLOWINSKI, J_ AND IVERSEN, L. L. (1966). Regional studies of catecholamines in the rat brain. I. The disposition of 'H-norepinephrine, 'H-dopamine and 'HDOPA in various regions of the brain. J. Neurochem. 13,655-669.
GOLLAMUDI, R., LopEz, M., LEAKEY, J., WEBB, P., AND SLIKKER, W., JR. (1988). Metabolism of 3,4-methylenedioxymethamphetamine (MDMA) by rat liver microsomes. Toxicologist 8, 200.
GREER, G. (1985). Using MDMA in psychotherapy. Advances 2, 57-59.
MOLLIVER, M. E., O'HEARN, E., BATTAGLIA, G., AND DE SOUZA, E. B. (1986). Direct intracerebral administration of MDA and MDMA does not produce serotonin neurotoxicity. Soc. Neurosci. Abstr. 12, 336.3.
NADLER, J. V., AND EVENSON, D. A. (1983). Use of excitatory amino acids to make axon-sparing lesions of the hypothalamus. In Methods in Enzymology (P. M. Conn, Ed.), Vol. 103, pp. 393-400. Academic Press, New York.
PAULE, M. G., REUHL, K., CHEN, J. J., ALI, S. F, AND SLIKKER, W. JR. (1986). Developmental toxicology of trimethyltin in the rat. Toxicol. Appl. Pharmacol. 84, 412417.
PAXINos, G., AND WATSON, C. (1982). The Rat Brain in Stereotoxic Coordinates. Academic Press, New York.
RICAURTE, G., BRYAN, L., STRAUSS, L., SEIDEN, L., AND SCHUSTER, C. (1985). Hallucinogenic amphetamine selectively destroys brain serotonin nerve terminals: Neurochernical and anatomical evidence. Sci ence 229, 986988.
SCALLET, A. C., ALI, S. F., HoLsoN, R. R.. IJFE, G. W., AND SLIKKER, W. JR. (1988). Neuropathological evaluation of MDMA (Ecstasy) by immunohistochemistry and a degeneration specific method. Neurotoxicology, in press.
SCHMIDT, C. J. (1987). Neurotoxicity ofthe psychedelic amphetamine, methylenedioxymethamphetamine. J. Pharmacol. Exp. Ther. 240,1-7.
SHULGIN, A. T., AND NICHoLs, D. E. (1978). Characterization of three new psychotominetics. In The Psychopharmacology of Hallucinogens(R. C. Stillman and R. E. Willete, Eds.), pp. 74-83, Pergamon Press, New York.
SLIKKER, W., JR., VORE, M., BAILEY, J. R., MEYERS, M., AND MONTGOMERY, C. (1983). Hepatotoxic effects of estradiol- I 3B-D-glucuronide in the rat and monkey. J. Pharmacol. F_%p. Ther. 2M 138-143.
SZEBENYi, E. S. (1970). Atlas of Macaca mulatta. Univcrsity Press, Rutherford, NJ.
WINER, B. J. (197 1). Statistical Principles in Experimental Design, 2nd ed., pp. 149-205. McGraw-Hill, New York.
Last Updated (Monday, 20 December 2010 19:45)












