MDMA: further evidence that its action in the medial prefrontal cortex is mediated by the serotonergic system
Drug Abuse
MDMA: further evidence that its action in the medial prefrontal cortex is mediated by the serotonergic system
Helen S. Pan and Rex Y. Wang
Department of Psychiatry and Behavioral Science, Putnam Hall, South Campus, State University of New York at Stony Brook, Stony Brook,
NY 11794 (U.S.A.)
Correspondence: R.Y. Wang, Department of Psychiatry and Behavioral Science, Putnam Hall, Room 147, South Campus, State University of New York at Stony Brook, Stony Brook, NY 11794-8790, U.S.A.
Key words: 5-Hydroxytryptamine; 3,4-Methylenedioxymethamphetamine; Medial prefrontal cortex; Single unit recording
Systemically administered (±)-MDMA (3,4-methylenedioxymethamphetamine, 'Ecstasy') suppressed the firing rates of the majority of neurons in the medial prefrontal cortex (mPFc). The responses of mPFc cells to (±)-MDMA is mimicked by (+)-MDMA but not (-)-MDMA. Furthermore, pretreatment with fluoxetine (a specific 5HT uptake blocker) but not GBR 12909 (a specifie dopamine uptake blocker) prevented the suppressant action of MDMA. These data support the notion that the 5HT system mediates (±)-MDMA's action.
MDMA (3,4-methylenedioxymethamphetamine, 'Ecstasy') is a recreational substance of abuse. It has been shown to interact with the brain serotonin (5hydroxytryptamine, 5-HT) and dopamine (DA) systems, although the DA system is less pronouncedly affected". MDMA potently depletes regional brain 5-HT content both acutely and chronically211. The lonl-term depletion of 5-HT is correlated with the development of 5-HT neurotoxicity 17 which could be prevented by treatment with 5-HT, antagonists'9. The short-term 5-HT content changes may be related to MDMA's behavioral and psychological effects. Interestingly, MDMAinduced acute behavior such as locomotion could be blocked by pindolol (5-HTIA.,3 and # receptor antagonist) but not by 5-HT2 receptor antagonists (Geyer et al., personal communication).
We have recently demonstrated that (±)-MDMA's suppressant action on the firing activity of medial prefrontal cortical (mPFc) cells was either abolished or markedly attenuated in animals whose brain 5-HT content was depleted by p-chlorophenylalanine (PCPA) but was not affected in animals whose brain dopamine (DA) content was depleted by a-methyl-p-tyrosine (AMPT)1-5. Moreover, the precursor of 5-HT, 5-hydroxytryptophan, but not the precursor of DA, L-DOPA, reinstated the suppressant action of MDMA in PCPA-treated rats . Additionally, our preliminary results show that the suppressant action of MDMA was reversed by metergoline (5-HT,,, receptor antagonist) and by granisetron (5-HT, receptor antagonist), although the latter's effect was less consistent. To further support the view that the 5-HT system is critical in mediating the action of MDMA, we report here that pretreating the animals with the 5-HT uptake blocker fluoxetine but not the DA uptake blocker GBR 12909 prevented the effect of (±)-MDMA. In addition, (+)-MDMA but not MDMA mimicked the action of (±)-MDMA.
Male Sprague-Dawley rats (200-250 g, Taconic Farms, NY) were anesthetized with chloral hydrate (400 mg/kg, i.p., Sigma) and placed in a stereotaxic frame. The tail vein was then cannulated with a 25 gauge needle for the administration of additional anesthetic or drugs. A burr hole was drilled above the mPFc (0.3-1.0 mm lateral and 11.0-11.5 mm anterior to Lambda) according to the atlas of Paxinos and Watson 16 to allow the lowering of a single barrel glass recording electrode (in vitro impedance of 2-4 MS2, filled with 2 M NaCl saturated with 1% Fast green dye). The effects of the individual isomers as well as racemic MDMA on the firing of spontaneously active mPFc neurons were studied using standard extracellular single unit recording techniques as described in detail elsewhere 26 . The mPFc is chosen because it is a brain region which is involved in cognition and the reward phenomenon and because we have characterized the physiological and pharmacological properties of 5-HT2 and 5-HT3 receptors in this region 2,3 . MDMA was given according to a regimen in which each dose doubled the previous cumulative dose until the cell was completely inhibited or the end of the dose-response regimen was reached (final cumulative dose: 25.6 mg/kg).
To obviate residual drug effects, only one cell was studied in each rat. The response of a mPFc cell was termed 'inhibited' or 'excited' when a drug decreased or increased the basal firing rate by --20%. At the end of each experiment, -30 pA of current was passed for 20 min through the electrode to eject Fast green dye and thus mark the location of the electrode tip. The rats were then perfused transcardially and the recording sites were verified histologically.
As shown previously, in control rats, (±)-MDMA predominantly inhibited the firing of spontaneously active mPFc neurons (Fig. 1). Specifically, (±)-MDMA inhibited the firing of the majority (16/19, 84%) and excited the firing of a minority (3/19, 16%) of the neurons (FiP. 2A) 15. Similarly, (+)-MDMA inhibited the firing of 9L , J 10%) and excited 1/10 (10%) mPFc cells (Fig. 2A). In contrast, (-)-MDMA inhibited the firing of 6/15 (40%) cells, excited 8/15 (53%) cells and had no effect on the remaining one (7%) cell (Fig. 2A). The profile of mPFc cells' responses to (±)-MDMA is statistically different (Logistic regression, P 0.05). In parallel, when the responses ofall the neurons were considered, the cumulative doseresponse curve of (-)-MDMA is shifted to the right with respect to that for (±)-MDMA (ANOVA and Student's Newman-Keul's test, P 0.05) (Fig. 213). The ICO values extrapolated from the curves were 17.1 mg/kg for (±)-MDMA and 6.5 mg/kg for (+)-MDMA. The IC,o value for (-)-MDMA cannot be determined because even at the highest cumulative dose, the cells on the average were not appreciably inhibited. When only the cells which were inhibited by MDMA were considered, the IC,, values for (±)-, (+)- and (-)-MDMA were 8.0 ± 2.3 (n = 15 ), 4.2 ± 1.1 (n = 9 ) and 15.6 ± 4.9 (n = 6 ) mg/kg, respectively.
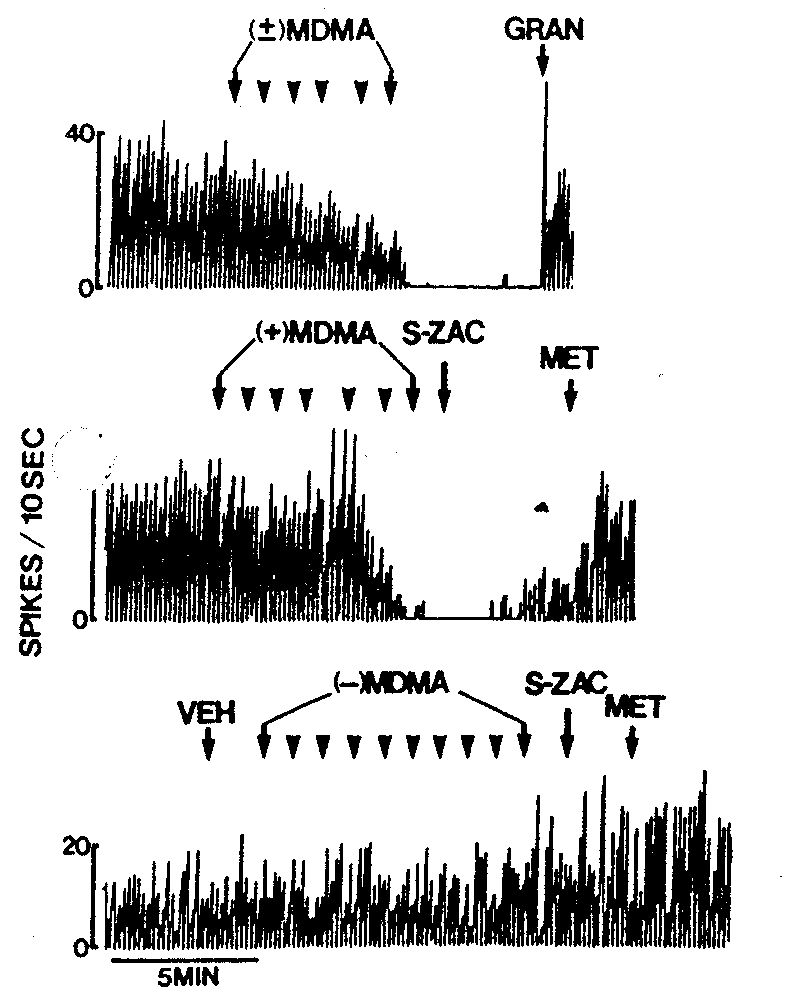
Fig. 1. Representative rate histograms showing the effect of (+)- and (-)-M13MA on the firing of spontaneously active mPFc neurons in normal animals. Top: this rate histogram shows the inhibitory effect of (±)-MDMA on a typical mPFc cell. Granisetron (GRAN, 1 mg/kg) reversed its effect. MDMA had a similar inhibitory action on the majority of mPFc neurons. Middle: (+)-MDMA similarly inhibited the firing of this particular mPFc neuron. S-Zacopride (S-ZAC, 0.1-0.4 mg/kg) partially and metergoline (MET, 0.1 mglkg) completely reversed its effect. The majority of mPFc neurons was similarly inhibited by (+)-MDMA. Bottom: (-)-M13MA had no effect on the majority of mPFc neurons as illustrated by this cell. S-ZAC (0.5-1 mg/kg) and MET (0.5-1 mg/kg) as well as vehicle had no effect on MDMA's action.
The effects of (±)-MDMA were reversed by the 5-HT receptor antagonists metergoline, granisetron and Szacopride (0.5-2 mg/kg, i.v.) although metergoline's action was the most consistent. When MDMA-induced inhibitory effect was considered, metergoline, granisetron and S-zacopride completely reversed its action in 11/25 (44%), 3/9 (33%) and 015 (0%) cells, respectively; partially reversed its action in 10/25 (40%), 2/9 (22%) and 3/5 (60%) cells; and did not reverse its action in 4/25 (16%), 4/9 (44%) and 2/5 (40%) cells. The MDMAinduced excitatory effect was completely reversed in 3/6 (50%), partially reversed in 1/6 (17%) and was not reversed in 2/6 (33%) mPFc cells by metergoline.
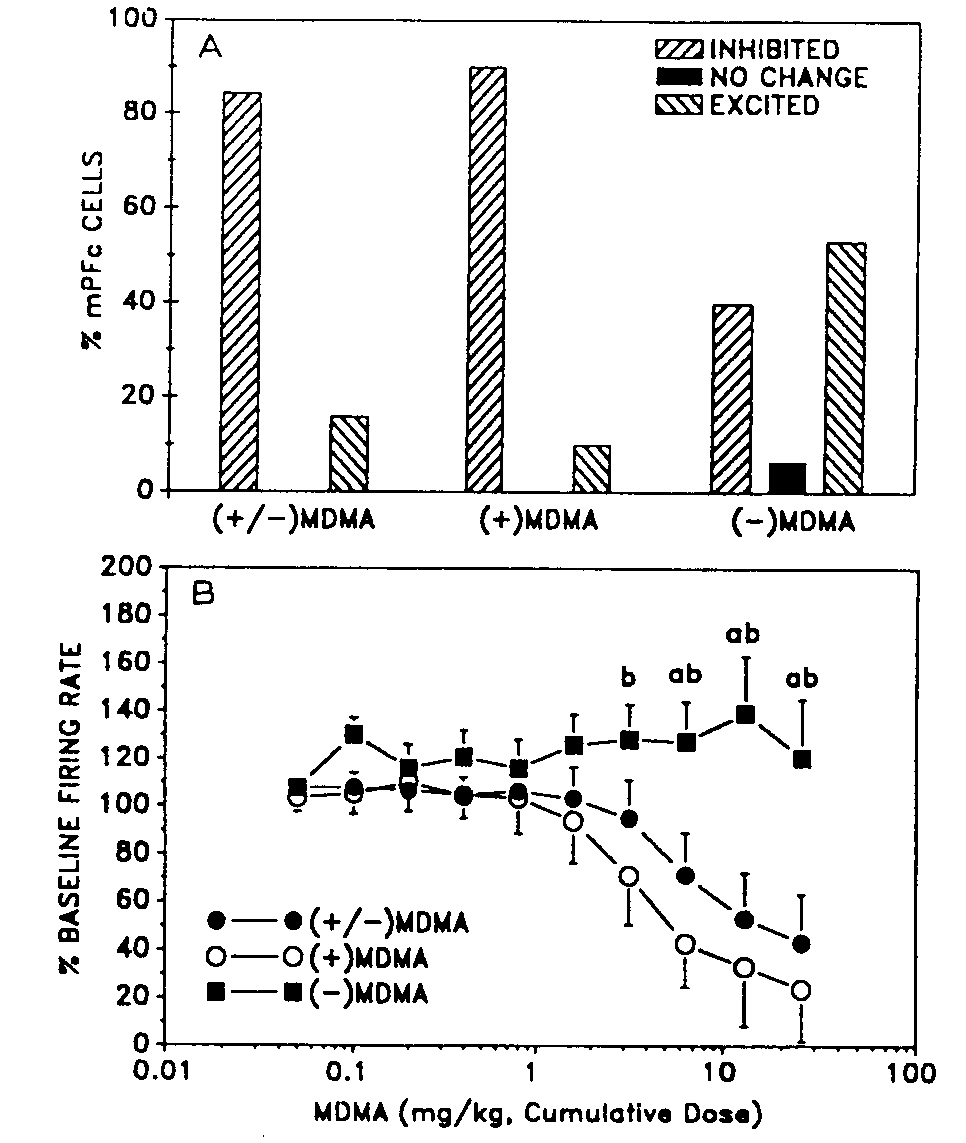
Fig 2. The effect of (±)-, (+)- and (-)-MDMA (25.6 mglkg, cumulative dose) on the firing activity of mPFc neurons in normal animals. A: (±)-MDMA inhibited the firing of 16/19 (84%) and excited 3/19 (16%) cells. (+)-MDMA inhibited 9110 (90%) and excited 1/10 (10%) mPFC cells. (-)-MDMA inhibited 6/15 (40%), excited 8/15 (53%) and had no effect on 1/15 (7%) mPFc cells. The distribution of responses for (±)-MDMA is the same as that for (+)-MDMA but is significantly different from that for ()-MDMA. B: the cumulative dose-response curves, including all cells studied, for (±)-, (+)- and (-)-MDMA. The curve for (-)-M13MA is significantly shifted to the right compared to those for (±)-MDMA and (+)-MDMA. a, P < 0.05, significantly different from (±)MDMA, b, P < 0.05, significantly different from (+)-MDMA. Vertical bars = S.E.M.
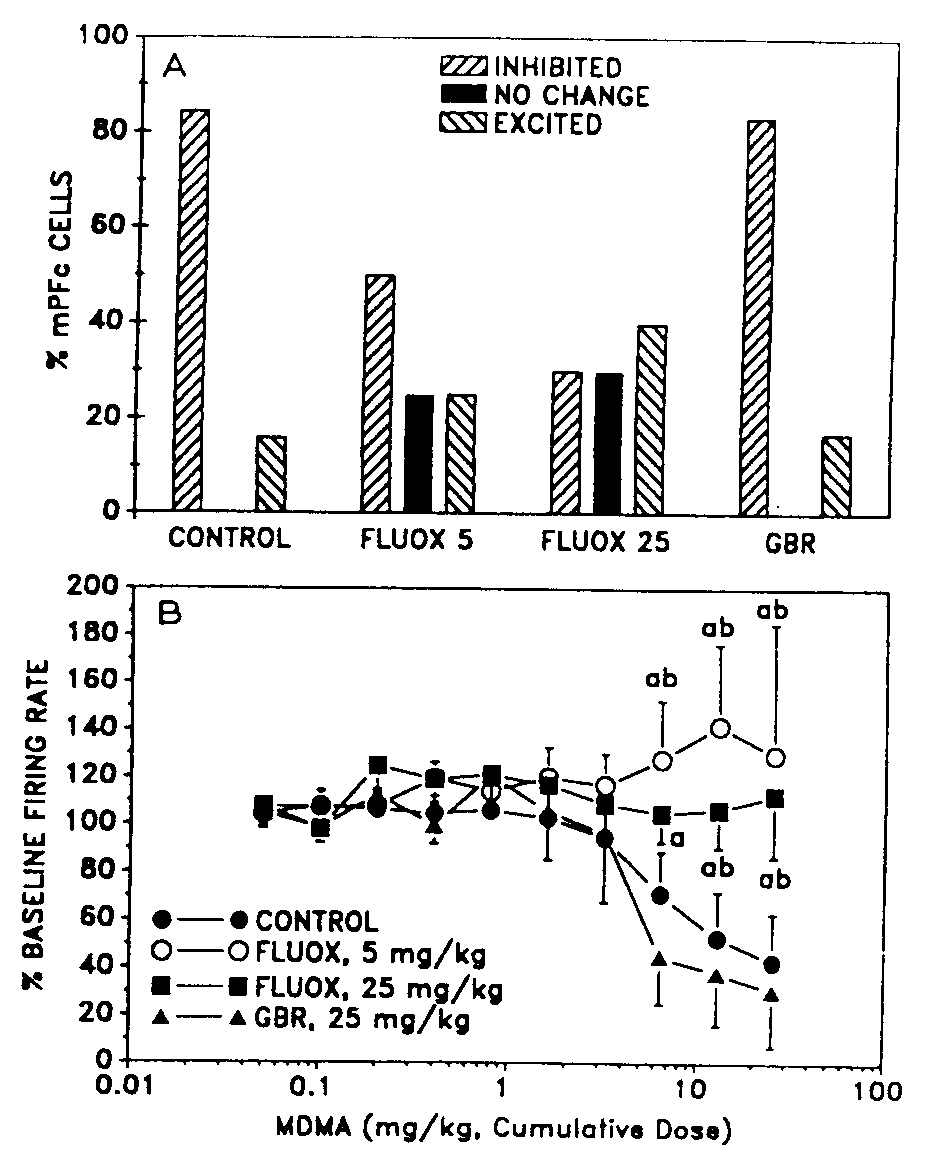
Fig. 3. The effect of fluoxetine (FLUOX) and GBR 12909 (GBR) treatment on mPFc cells' response to (±)-MDMA. A: in animals pretreated with FLUOX (5 or 25 mg/kg, i.p.) (±)-MDMA either excited or had no effect on more mPFC cells compared to controls. In contrast, GBR pretreatment did not have comparable effects. 13: cumulative dose-response curves of MDMA's effect on the firing rates of all the mPFc cells studied revealedthat FLUOX pretreatment significantly shifted (±)-MDMA's dose-response curves to the right compared to those in controls and GBR pretreated animals. a, P < 0.05, significantly different from controls. b, P < 0.05, significantly different from GBR-pretreated animals. Vertical bars = S.E.M.
In animals treated with 5 mg/kg fluoxetine intraperitoneally 15-30 min before the electrophysiological experiment, 4/8 (50%) of mPFc cells were inhibited, 2/8 (25%) cells were excited and the remaining 2/8 (25%) cells were not affected by the drug (Fig. 3A). This distribution narrowly missed being significantly different from that produced by (±)-MDMA in controls (Logistic regression, P > 0.05). In animals treated with 25 mg/kg fluoxetine intraperitoneally 15-30 min before recording had begun, 3/10 (30%) cells were inhibited, 3/10 (30%) cells were excited and the remaining 4/10 (40%) cells were unaffected (Fig. 2A). This profile of response is significantly different from that seen in controls (Logi,, regression, P 0.05) (Fig. 2A). The cumulative close-respons curves describing (±)-MDMA's effect on mPFc cell after the aforementioned treatments are shown in Fi~ 3B. IC5, values extrapolated from these curves were 17 mg/kg for controls and 11.6 mg/kg for animals treatc with GBR 12909. 1C50 values could not be determined fo animals treated with fluoxetine because even at th highest dose (25.6 mg/kg) studied the cells on the averaF were inhibited by less than 50% of baseline. Overall, th dose-response curves for controls and the GBR 12909 treated animals were not different (ANOVA and Stu dent's Newman-Keuls's aest , P > 0.05), whereas thos for controls were significantly different from animal which had received either 5 or 25 mg/kg fluoxetin~ (ANOVA and Student's Newman-Keul's test, P < 0.05 1030 min before recording began.
The present results show that (±)-MDMA suppresse the firing of the majority of mPFc neurons. The dos which suppresses the firing by 50% is slightly higher tha: the typical dose used by human users (1-5 mg/kg, p.o but is in agreement with doses given to monkeys mg/kg, i.v., LD,(, = 22 mg/kg) and rats (1-5 mg/kg, i.\ up to 300 mg/kg, p.o., LD,(, = 32 mg/kg, i.p.)21.
Our data also show that MDMA's suppressant effect i prevented or markedly attenuated by fluoxetine but n( GBR 12909. Our finding is in concordance with the ide that the effect of (±)-MDMA is mediated via the 5-H but not DA system. For instance, we and others ha\~ shown that MDMA-induced electrophysiological el fects' 2.15.23 and locomotion 6 are prevented by pretreatinl~ the animals with PCPA or fluoxetine but not AMPI Taken together, the data indicate that the effect o MDMA is dependent on its 5-HT-releasing and uptake blocking properties, actions which have been docu mented by many biochemical studieS20,24,25.
Our finding that (+)-MDMA is more effective thai (-)-MDMA in inhibiting the firing of mPFc cells I consistent with the report that of the two isomers, th( (+)enantiomer has a more potent action in releasin,~ 5-HT 20. Similarly, Spouse et al .23 found that (+)-MDM/I inhibits the firing of dorsal raphe neurons by releasin,~ 5-HT and that these cells are 2 to 3 times more sensitiv( to the action of (+)-MDMA than the (-)-isomer. Studie using various behavioral paradigms have also detecte differences in the action of the two enantiomers. Fo example, (+)-MDMA is more potent than (-)-MDMA ii drug discrimination tests7.18, in producing hyperthermia and in eliciting stereotyped behaviorslo, although this last behavior has not been uniformly observed by others 9,14,27 using the racemate
MDMA has been reported to have a micromolar affinity for postsynaptic 5-HT receptors, especially the 5-HT2 receptor subtype 4 . However, the (-)-isomer appears to have a higher affinity than the (+)-isorner at this binding site 13. Interestingly, 5-HT, receptors have been implicated in the action of hallucinogens and the (-)enantiomer of the hallucinogens is the more active one. Our finding that (+)-MDMA is the more active isomer may be related to the fact that MDMA has little or no hallucinogenic properties and indicates that MDMA's mechanism of action may be mediated by 5-HT sites oth,~r than (or in addition to) the postsynaptic 5-HT, rk:,)$ dtors.
In this study, MDMA's suppressant action was reversed by the 5-HT3 receptor antagonists S-zacopride, granisetron and the 5-HT,,2 receptor antagonist metergoline. However, the antagonism produced by S-zacopride and granisetron was less consistent than metergoline. The data suggest that both 5-HT, and 5HT3 receptors may play a role in mediating MDMA's suppressant effect in the mPFc. Consistent with our finding, Geyer et al. (personal communication) have presented preliminary results showing that 5-HT, receptors are important in mediating the MDMA-induced locomotor activity as pindolol effectively blocks MDMA's action. On the other hand, it is tempting to speculate that 5-HT2 receptors contribute to the occasional excitatory effect produced by MDMA as stimulation of this receptor subtype has been reported to excite or facilitate the excitatory action of glutamate on neurons in the frontal cor-tex'.5. Obviously, more research is needed to further elucidate the involvement of the specific 5-HT, receptor subtypes in the action of MDMA. In summary, although several lines of evidence suggest that MDMA, in addition to the 5-HT system, activates the DA syste M8,20,22,27,28, our data indicate that 5-HT, but not DA, plays an important role in MDMA's action in the mPFc. Nevertheless, it is likely that MDMA may have a more substantial effect on the DA-systern in other brain areas rich in DA content and innervation.
We wish to thank SuXin Tian for confirming the recording sites histologically and for working on the photographs. We also wish to thank NIDA for the supply of (±)-MDMA and its optical isomers and Eli Lilly for the donation of fluoxetine HC1. This work was supported by USPHS Grants MH-41440 and MH-00378 (RSDA) to R. Y. W.
I Anderson, G.M., Braun, G., Braun, U., Nichols, D.E. and Shulgin, A.T., Absolute configuration and psychotornimetic activity, NIDA Res. Monogr., 22 (1978) 815.
2 Ashby Jr., C.R., Edwards, E., Harkins, K. and Wang, R.Y., Characterization of 5-hydroxytryptamine3 receptors in the medial prefrontal cortex: a microiontophoretic study, Eur. J. Pharmacol., 173 (1989) 193-190.
3. Ashby Jr., C.R., Jiang, L.H., Kasser, R.J. and Wang, R.Y., _ctrophysiological characterization of 5-hydroxytryptamine2 receptors in the medial prefrontaJ cortex: a microiontophoretic study, J. Pharmacol. Exp. Ther., 252 (1990) 171-178.
4 Battaglia, G., Brooks, B.P., Kulsakdinun, C. and De Souza, E.B., Pharmacological profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites, Eur.J. Pharmacol., 149 (1988) 159-163.
5. Davies, M.F., Deisz, R.A_ Prince, D.A. and Peroutka, S.J., Two distinct effects of 5-hydroxytryptamine on single cortical neurons, Brain Research, 423 (1987) 347352.
6 Geyer, M.A., Callaway, C.W. and Nichols, D.E., Serotonin release is responsible for the locomotor hyperactivity in rats induced by derivatives of amphetamine related to MDMA, Programme and A bstr. for the 2nd 1UPHA R satellite meeting on serotonin, 1990, p. 112.
7 Glennon, R.A., Little, P.J., Rosecrans, J.A. and Yousif, M., The effect of MDMA ('Ecstasy') and its optical isomers on schedule-controlled responding in mice, Pharmacol. Biochem. Behav., 26 (1987) 425-426.
8 Glennon, R.A. and Misenheimer, B.R., Stimulus effects of N-monoethyl-l-(3,4-methylenedioxyphenyl)-2-aminopropane (MDE) and N-hydroxy-l-(3,4-methylenedioxyphenyl)-2-amino- propane (N-OH MDA) in rats trained to discriminate MDMA from saline, Pharmacol. Biochem. Behav., 33 (1989) 909-912.
9 Gold, L.H., Geyer, M.A. and Koob, G.F., Neurochcmical mechanisms involved in behavioral effects of amphetamines and related designer drugs, NIDA Res. Monogr., 94 (1989) 101-126.
10 Hiramatsu, M., Nabeshima, T., Kameyama, T., Maeda, Y. and Cho, A.K., The effect of optical isomers of 3,4-methylenedioxymethamphetamine (MDMA) on stereotyped behavior in rats, Pharmacol. Biochem. Behav., 33 (1989) 343-347.
11 Johnson, M.P., Hoffman, A.J, and Nichols, D.E., Effects of the enantiomers of MDA, MDMA, and related analogues on [3 HIserotonin and ['Hldopamine release from superfused rat brain slices, Eur. J. Pharmacol., 132 (1986) 269-276.
12 Kelland, M.D., Freeman, A.S. and Chiodo, L.A., (±)-3, 4-Methylenedioxymethamphetamine-induced changes in the basal activity and pharmacological responsiveness of nigrostfiatal dopamine neurons, Eur. J. Pharmacol., 169 (1989) 11-21.
13 Lyon, R.A, Glennon, R.A. and Titter, M., 3,4-Methylenedioxymethamphetamine (MDMA): stereoselective interactions at 5-HT, and 5-HT, receptors, Psychopharmacology, 88 (1986) 525-526.
14 Matthews, R.T., Champney, T.H. and Frye, G.D., Effects of (±)3,4-methylenedioxymethamphetamine (MDMA) on brain doparnmergic activity in rats, Pharmacol. Biochem. Behav., 33 (1989) 741-747.
15 Pan, H.S. and Wang, R.Y., The action of (±)-MDMA is mediated through the serotonin system, Brain Research, in press.
16 Paxinos, G. and Watson, C., The Rat Brain in Stereotaxic Coordinates, Academic Press, New York, 1988.
17 Ricaurte, G.A., DeLanney, L.E., Irwin, 1. and Langston, J,W., Toxic effect of MDMA on central serotonergic neurons in the primate: importance of route and frequency of drug administration, Brain Research, 474 (1988) 359-363.
18 Schechter. M.D., MDMA as a discriminative stimulus: isomeric comparisons, Pharmacol. Biochem. Behav., 27 (1987) 41-44.
19 Schmidt, C.J., A role for 5-HT. receptors in the serotonergic deficits produced by the psychedelic amphetamine analogue, methylenedioxymethamphetamine (MDMA), Programme and Abstr. for the 2nd lUPHAR satellite meeting on serotont . n, 1990,
20 Schmidt, C.J., Levin, J.A. and Lovenherg, W., In vitro and in vivo neurochernical effects of methylenedioxymethamphetamine on striatal monoaminergic systems in the rat brain, Biochem. Pharmacol., 36 (1987) 747-755.
21 Shulgin, A.T., History of MDMA. In SJ. Peroutka (Ed.), Ecstasy,: The Clinical, Pharmacological and Neuroloxicological Effects of the Drug MDMA, Kluwer, Boston, 1990, pp. 1-20.
22 Spanos, L.J. and Yamamoto, B.Y., Acute and subchronic effects of methylenclioxymethamphetamine [(±)MDMAI on locomotion and serotonin syndrome behavior in the rat, Pharmacol. Biochem. Behav- 32 (1989) 835-840.
23 Sprouse, J.S.. Bradberry, C.W., Roth, R.H. and Aghajanian, G.K., MDMA (3,4-Methylenedioxymethamphetamine) inhibits the firing of dorsal raphe neurons in brain slices via release of scrotonin, Ear. J. Phannacol., 167 (1989) 375-383.
24 Steele, T.D., Nichols, D.E. and Yim, G.K.W., Stereochemical effects of 3,4-methylenedioxymethamphet~imine (MDMA) an( related amphetamine derivatives on inhibition of uptake ol ["Himonoamines into synaptosomes from different regions of rat brain, Biochem. Pharinacol., 36 (1987) 2297-2303.
25 Stone, D.M., Merchant, K,M., Hanson, G.R. and Gibb, J.W., Immediate and long-term effects of 3,4-methylenedioxymetham
phetamine on serotonin pathways in brain of rat, Neuropharmacology, 26 (1987) 1677-1683. 1
26 Wang, R.Y. and Aghajanian, G.K., Inhibition of neurons in the amygdala by dorsal raphe stimulation: mediation through a direct serotonergic pathway, Brain Research, 120 (1977) 85102,
27 Wilkerson, G. and London, E.D., Effects of methylenedioxymethamphetamine on local cerebral glucose utilization of the rat, Neuropharmacology,28 (1989) 1129-1138.
28 Yamamoto, B.K. and Spanos, L.J., The acute effects ot met hylenedioxymethamphetarnine on cloparnine release in the awake-behaving rat, Ear. J. Pharmacol., 148 (1988) 195203,
Last Updated (Monday, 20 December 2010 19:40)












