Neuropharmacology and Neurotoxicity
Drug Abuse
neuro?report
EXTRACELLULAR recordings were used to determine the effects of cannabinoids on the activity of dopamine neurons within the ventral tegmental area (VTA) and substantia nigra pars compacta (SNC). Systemic administration of the natural psychoactive cannabinoid 09tetrahydrocannabinol (09-THC) and the synthetic cannabimimetic aminoalkylindole WIN 55,212-2 produced dose-dependent increases in firing rate and burst firing in both neuronal populations. These effects appear to be specific as the non-psychoactive cannabidiol and the inactive enantiomer WIN 55,212-3 failed to alter either parameter of neuronal excitability. Furthermore, dopamine neurons in the VTA were more sensitive than those in the SNC to the stimulatory actions of 09-THC. These results may provide a mechanism by which psychoactive cannabinoids increase extracellular dopamine levels in mesolimbic and striatal tissues, and thereby contribute to the reinforcing effects of marijuana.
Key words: Cannabinoids; Electrophysiology; Marijuana; Substantia nigra; Ventral tegmental area
NeuroReport 8, 649–652 (1997)
Cannabinoids excite
dopamine neurons in
the ventral tegmentum
and substantia nigra
Edward D. French,CA Kathryn Dillon and Xiaofang Wu
Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ 85724, USA
CACorresponding Author
Introduction
A preponderance of experimental data obtained in animals strongly supports the notion that the reinforcing properties of commonly abused drugs are mediated through the mesolimbic–mesocortical dopamine system originating in the midbrain ventral tegmental area (VTA).1,2 Marijuana, a highly abused illicit substance would be suspected, therefore, to alter dopamine activity in reward relevant circuits in the brain, in particular the mesolimbic pathway from the VTA to the nucleus accumbens. Prior studies have shown that the psychoactive constituent of marijuana, 09-tetrahydrocannabinol (09-THC), increases extracellular levels of dopamine in the accumbens and striatum, and induces amphetamine-like ipsiversive turning in rats with unilateral nigrostriatal lesions.3–6 Thus, it has been suggested that 09-THC acts through a presynaptic site of action possibly in the manner of a dopamine reuptake inhibitor. An alternative mechanism by which 09-THC could augment dopamine neurotransmission has not been fully explored, namely 09-THC-induced changes in dopamine cell firing. Therefore, the present study was designed to determine the effects of systemic administration of 09-THC and the non-psychoactive cannabidiol (CBD) on the activity of single dopamine neurons within the VTA and substantia nigra pars compacta (SNC). The effects of the synthetic cannabimimetic aminoalklyindole WIN 55,212-2 and its inactive enantiomer WIN 55,212-3 were also assessed and compared with those of the natural cannabinioids.
© Rapid Science Publishers
Materials and Methods
Adult male Sprague–Dawley rats weighing 250–350 g were used in all experiments. All animals were housed under a light-dark schedule (07.00–19.00 h) with constant room temperature and free access to food and water. All experimental and surgical manipulations were carried out in accordance with a University of Arizona IACUC approved protocol.
Chloral hydrate (350 mg kg–1, i.p.) was used for the induction and maintenance of anesthesia throughout the recording period. The preparation of the animal for i.v. drug injections and a detailed description of the electrophysiological techniques for recording from VTA and SNC dopamine neurons have been described elsewhere.7 Inter-spike interval histograms for computing burst activity were constructed off-line from 500 consecutive spikes preceding the onset of the next injection. The criteria used for the definition of bursting parameters have been detailed elsewhere.7 VTA and SNC recording sites were made at the following coordinates relative to bregma: posterior 5–5.5 mm, and lateral 0.5–1.0 (VTA) and 1.2–2.0 mm (SNC).
All drugs were injected i.v., and injections were separated by intervals sufficient to collect at least 500 spikes, which in most cases was 2–4 min. Drugs were administered in a cumulative dosing paradigm according to the following protocol: 09-THC and CBD: 0.125, 0.25, 0.5, 1, 2 and 4 mg kg–1, for a total cumulative dose of 7.875 mg kg–1, and WIN 55212-2 and -3: 0.0125, 0.025, 0.05, 0.1, 0.2 and 0.4 mg kg–1, for a total cumulative dose of 0.7875 mg kg–1. Only
Vol 8 No 3 10 February 1997 649
E. D. French, K. Dillon and X. Wu
one cell per animal was tested. A9-THC and CBD were obtained from Sigma Chemical Co. and WIN 55212-2 and -3 from Sterling Winthrop. All drugs were prepared in the following vol/vol vehicle: 10% Tween 80, 20% dimethylsulfoxide (DMSO) and 70% distilled water. An aliquot of stock A9-THC (100 mg A9-THC ml–1 ethanol) was placed in a vial and evaporated to dryness under a stream of argon gas. DMSO was then added and vortexed, followed by Tween80 and vortexed, and lastly water and vortexed. Solutions were prepared fresh for each experiment.
Results
A total of 63 dopamine neurons in the VTA and SNC was assessed for their response to A9-THC (n = 23), cannabidiol (n = 9), WIN 55,212-2 (n = 21), WIN 55,212-3 (n = 5) and vehicle (n = 5).
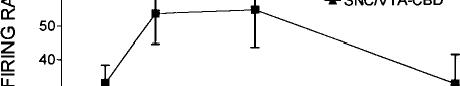
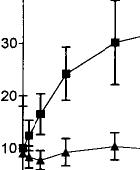
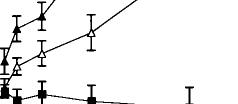
Systemic injections of A9-THC produced significant (p < 0.01) dose-dependent increases in dopamine cell firing in both VTA and SNC with maximum increases of 55% in the VTA and 28% in the SNC (Fig. 1, top). An examination of the dose–response curves further reveals that VTA neurons required about one-sixth the dose of A9-THC to produce a level of excitation (20%) comparable with that seen in the SNC. These effects appear to be specific to the psychoactive cannabinoid since the non-psychoactive CBD failed to affect these parameters of dopamine cell excitability, even at higher doses (data not shown). The vehicle of DMSO/Tween-80/water was ineffective, producing a maximum change in rate of only –7%. Cannabinoid-induced changes in the number of action potentials contained in bursts were also dose-dependently increased in the VTA from a basal level of 9.7% to 34.5% (Fig. 1, bottom). Bursting in the SNC was virtually unchanged, however, from a baseline of 9.6% to 10.3% (Fig. 2).
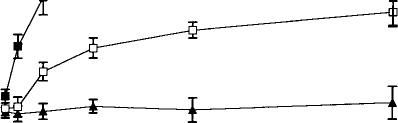
The electrophysiological response of dopamine neurons to the cannabimimetic enantioselective WIN 55,212-2 was a significant (p < 0.01 by one-way ANOVA) excitation with firing rates in the VTA and SNC increasing by 61.5% and 58.5%, respectively (Fig. 2). Burst firing also increased in both regions from 6.6% to 22.5% in the SNC and from 21% to 31% in the VTA. Again, SNC neurons were less sensitive than those in the VTA, requiring about 2.5 times more WIN 55,212-2 to produce a comparable change (40%) in rate. These effects also appear to be specific since the inactive isomer WIN 55,212-3 failed to change VTA activity; SNC neurons were not tested with the inactive enantiomer.
80
70
60
z
W 20
(9 z
Q 10U
30
0
-10
0
1
2
5
6
7
8
5040
--VTA -A- SNC
0 z
H
U)
a! m
0
60
40
20
-A-VTA-WIN 55,212-2 -~-SNC-WIN 55,212-2 -wVTA-WIN 55,212-3
1 2 3 4 5 6 7
CUMULATIVE DOSE (mg/kg, i.v.)
FIG. 1. Dose-dependent effects of the psychoactive cannabinoid 09THC on firing rate (top graph) and burst firing (bottom graph) in VTA and SNC dopamine neurons. One-way ANOVA with dose as the repeated measure found significant effects on firing rate in both VTA (F = 6.7, p < 0.01, df 5,67) and SNC (F = 15.1, p < 0.01, df 5,59). Moreover, two-way ANOVA with repeated measures found a significant difference between 09-THC firing rates in VTA vs those in SNC (F = 28.7, p < 0.01, df 1,5). The non-psychoactive CBD was ineffective both in the VTA and SNC and the results were therefore combined into a single treatment group (F = 0.3, NS, 6,62).
FIG. 2. Dose–response effects of the active (WIN 55,212-2) and inactive (WIN 55,212-3) aminoalkylindoles on firing rates of VTA and SNC dopamine neurons. One-way ANOVA with dose as the repeated measure showed that WIN 55,212-2 elicited significant increases in firing rates in both VTA (F = 9.7, p < 0.01, df 5,59) and SNC (F = 10.2, p < 0.01, df 5,49). WIN 55,212-3 was ineffective in VTA (F = 0.2, NS, df 5,29) and, therefore, not tested in SNC.
3
-20 0.0
0.2 0,4 0.6
CUMULATIVE DOSE (mg/kg, i.v.)
08
650 Vol 8 No 3 10 February 1997
neuro?report
Discussion
The present results show that 09-THC, the psychoactive ingredient in marijuana, can excite midbrain dopamine neurons, in particular those in the VTA which comprise the mesolimbic–mesocortical pathways. Since these dopaminergic circuits are known to play a pivotal role in mediating the reinforcing effects of most drugs of abuse, the increased dopamine drive elicited by the cannabinoids could underlie the abuse property of marijuana.
These data also provide an alternative explanation for the observed increases in extracellular levels of dopamine following the administration of cannabinoids.8 It had been speculated that cannabinoidinduced increases in dopamine release were mediated through a presynaptic mechanism akin to that seen with dopamine reuptake blockers such as nomifensine.4 Dopamine reuptake inhibitors inhibit VTA and SNC firing, however. Therefore, the increased incidence of firing and bursting observed in the present study could provide an alternative mechanism by which cannabinoids increase extracellular dopamine levels. Burst activity in particular has been shown to markedly augment transmitter release.9 Furthermore, the 30% increase in VTA dopamine firing observed here at 1 mg kg–1 would appear to correspond to the 20% increase in dopamine release measured in the nucleus accumbens following the same dose of THC.8 The differences in the magnitude of change may best be explained by the i.v. vs i.p. routes of administration used in these two studies. Also, the doses leading to dopamine neuronal stimulation and mesolimbic dopamine release are relevant to human cannabinioid pharmacology. Nevertheless, it still remains to be determined whether the euphoria, dysphoria, or even psychotic-like symptoms that have been associated with marijuana use are the result of cannabinoidevoked activation of dopaminergic mesolimbic– mesocortical pathways.
In some respects the cannabinoid effects reported here bear a resemblance to the biochemical and electrophysiological changes observed in VTA dopamine neurons following the hallucinogen phencyclidine (PCP).7,10 Unlike PCP, however, psychoactive cannabinoids do not involve a site of action within the Nmethyl-D-aspartate-ion channel complex found on
VTA dopamine neurons.11,12 Furthermore, since
cannabinoid receptors do not appear to reside on dopamine cell bodies, the effects of 09-THC and WIN 55,212-2 would be more likely to occur through an alteration of transmitter(s) afferent to the VTA and SNC dopamine neurons.13 One possiblity would be cannabinoid-induced inhibition of local circuit -y-aminobutyric acid neurons within the VTA leading to a disinhibition of dopamine cell firing. However,
the finding that injections of 09-THC directly into the VTA fails to produce any extracellular dopamine changes in the nucleus accumbens argues against this mechanism.14 This might also rule out a direct stimulatory effect of cannabinoids on dopamine neurons. Ongoing studies in midbrain slice preparations will help determine whether the cannabinoid-induced excitations result from direct actions on dopamine neurons, local circuit neurons, or other VTA afferents.
The present findings that psychoactive cannabinoids stimulate midbrain dopamine neurons are also of interest given the fact that there appear to be few cannabinoid binding sites in either VTA or SNC.13 Nevertheless, an examination of autoradiograms of cannabinoid receptor binding of [3H]CP-55940 clearly shows labeling of cells in the VTA15. Furthermore, cells displaying low levels of hybridization to the mRNA for the cannabinoid receptor have been visualized throughout the substantia nigra and VTA.16 In addition, there are areas in the CNS where there is a mismatch between cannabinoid receptor density and the extent of metabolic effects of cannabinoids as revealed through 2-deoxyglucose auto
radiography.17
A surprising result of the present study was the apparent lack of sensitivity of dopamine neurons in the SNC compared to those in the VTA. It is wellknown that cannabinoids can produce marked catalepsy presumably by alterations of nigro-striatal dopamine release.5 The present data, however, would lead to the conclusion that this behavioral effect is likely not mediated through cannabinoid-induced stimulation of SNC neurons, since firing rates increased less than 30%, burst firing only 7% above baseline levels, with maximum effects on firing occurring at an i.v. dose of 8 mg kg–1. This dose of 09THC is considerably greater than that required to elicit catalepsy. Others have also reported that limbic structures are more sensitive than the striatum to 09THC-induced increases in dopamine metabolism.18 Also, the discriminative stimulus effects of 09-THC occur at doses five-fold less than those producing catalepsy.19 Whether the neurobiological underpinnings for cannabinoid drug discrimination and reinforcement share a common mesolimbic dopamine pathway is unclear. This differential sensitivity of midbrain dopamine neurons is not unique to 09THC, as other reinforcing drugs also produce larger changes in firing rate in VTA than in the SNC.20
It is generally accepted that the synthetic aminoalkyindole compound WIN 55,212-2 acts at the same receptor site as the natural cannabinoid moeities.21 In a battery of behavioral tests this drug produces the classic behavioral changes shown to occur following the administration of 09-THC.22 In the present
Vol 8 No 3 10 February 1997 651
E. D. French, K. Dillon and X. Wu
study, WIN 55,212-2 produced a similar magnitude of firing of VTA dopamine neurons to that seen with 09-THC but at doses generally 10-fold less. As with 09-THC, the increased firing rates were accompanied by increases in burst firing. Therefore, one might predict that WIN 55,212-2 would produce effects on dopamine release comparable to 09-THC. Notably, SNC neurons seemed more responsive to WIN than to 09-THC, and at the highest doses tested WIN 55,212-2 did not attenuate the increased firing rate as seen with 09-THC. These apparent differences between the cannabinoid compounds cannot be readily explained. Nevertheless, the effects of WIN 55,212-2 are considered specific since the inactive enantiomer WIN 55,212-3 failed to stimulate dopamine cell firing.
Conclusion
These data show that behaviorally active cannabinoids markedly increase both the firing rate and bursting activity of midbrain dopamine neurons. This mechanism may account for the increases in extracellular mesolimbic dopamine levels observed in in vivo microdialysis experiments. Midbrain dopamine neuronal systems are intimately involved in appetitive behaviors and as such may underly the learning and execution of goal directed behaviors.23 Thus, it is conceivable that the abuse potential of marijuana
may be subserved by cannabinoid-induced activation of the neuronal elements comprising this mesolimbic system.
References
1. Wise RA. Pharmacol Ther 35, 227–263 (1987).
2. Koob GF. Trends Pharmacol Sci 13, 177–184 (1992).
3. Ton JMNC, Gerhardt GA, Friedemann M et al. Brain Res 451, 59–68 (1988). 4. Gardner EL and Lowinson JH. Pharmacol Biochem Behav 40, 571–580
(1991).
5. Souilhac J, Poncelet M, Rinaldi-Carmona M et al. Pharmacol Biochem Behav
51, 3–7 (1995).
6. Sakurai-Yamashita Y, Ohta H, Shimazoe T et al. Life Sci 37, 2181–2185
(1985).
7. French ED, Mura A and Wang T. Synapse 13, 108–116 (1993).
8. Chen J, Paredes W, Lowinson J et al. Neurosci Lett 129, 136–140 (1991). 9. Gonon FG and Buda MJ. Neuroscience 14, 765–774 (1985).
10. Bowers MB Jr and Morton JB. Prog Neuro-Psychopharmacol Biol Psychiatry
18, 961–964 (1994).
11. Seigenbaum JJ, Bergmann F, Richmond SA et al. Proc Natl Acad Sci USA
|
86, 9584–9587 (1989). |
||
|
12. |
Wang T and French ED. Synapse 13, 270–277 (1993). |
|
|
13. |
Herkenham M, Lynn AB, de Costa BR et al. Brain Res 547, 267–274 |
(1991). |
|
14. |
Chen J, Marmur R, Pulles A et al. Brain Res 621, 65–70 (1993). |
|
|
15. |
Herkenham M, Lynn AB, Johnson MR et al. J Neurosci 11, 563–583 |
(1991). |
|
16. |
Matsuda LA, Bonner TI and Lolait SJ. J Comp Neurol 327, 535–550 |
(1993). |
|
17. |
Margulies JE and Hammer RP Jr. Eur J Pharmacol 202, 373–378 (1991). |
|
|
18. |
Bowers MB Jr and Hoffman FJ Jr. Brain Res 366, 405–407 (1986). |
|
|
19. |
Prescott WR, Gold LH and Martin BR. Psychopharmacology 107, 117–124 |
|
|
(1992). |
||
|
20. |
Kalivas PW. Brain Res Rev 18, 75–113 (1993). |
|
|
21. |
D’Ambra TE, Estep KG, Bell MR et al. J Med Chern 35, 124–135 (1992). |
|
|
22. |
Compton DR, Gold LH, Ward SJ et al. J Pharmacol Exp Ther 263, 1118–1126 |
|
(1992).
23. Mirenowicz J and Schultz W. Nature 379, 449–451 (1996).
ACKNOWLEDGEMENT: This work is supported through Grant DA 09025 from the National Institute on Drug Abuse.
Received 30 August 1996; accepted 17 October 1996
General Summary
Electrophysiological recordings from the midbrain of the rat were used to study the effects of cannabinoid drugs on single dopamine neurons in the ventral tegmentum and substantia nigra pars compacta. The natural psychoactive constituent of marijuana, A9-tetrahydrocannabiniol and the synthetic cannabinoid-like drug WIN 55,212-2 increased two parameters of neuronal excitability, firing rate and burst firing. The non-psychoactive ingredient in marijuana cannabidiol and the non-cannabinoid synthetic WIN 55,212-3 failed to alter either electrophysiological measure. These results provide a mechanism by which cannabinoids increase dopamine activity in limbic and striatal structures. Furthermore, this increase in dopamine function in limbic regions may partially underlie the reinforcing and abuse liability properties of marijuana.
652 Vol 8 No 3 10 February 1997
| Index |
| Index |
Last Updated (Monday, 20 December 2010 15:50)












