Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats
Drug Abuse
BIOCH[MICA ET BIOP]1YSIC. A(_'
ELSEVIER
Biochimica et Biophysica Acta 1407 (1998) 205-214
Changes in cannabinoid receptor binding and mRNA levels in several
brain regions of aged rats
F. Berrendero a, J. Romero a, L. Garcia-Gil a, 1. Suarez b. P, De la Cruz a.
J.A. Ramos a, J.J. Fernandez-Ruiz a.*
Insturao Comphaense de Drogodependencias, Departamento de Binquivriea Y Biologic Molecular, Facultad de Medicirra, t rrirersidud Complutense, 28040-Madrid, Spain " Departamento de Biologic Celular Y Genetica, Facultad de Ciencicrs, Universidad de A1ca/a, 28871-Madrid. Spain
Received 3 June 1998; accepted 10 June 1998
Abstract
We have recently found that cannabinoid receptor binding and gene expression markedly decreased in extrapyramidal structures of aged rats. The present study was designed to analyze the possible existence of similar aging-induced changes in cannabinoid receptor binding and gene expression in brain regions other than extrapyramidal areas. but that also contain a significant population of cannabinoid receptors, such as the cerebellum, hippocampal structures.. limbic and hypothalamic nuclei, the cerebral cortex and others. To this end, we analyzed cannabinoid receptor binding, using autoradiography. and cannabinoid receptor mRNA levels, using in situ hybridization, in slide-mounted brain sections obtained from young (3 month old) and aged ( ? 2 year old) rats. Results were as follows. In the cerebellum, aged rats exhibited a marked decrease in cannabinoid receptor binding in the molecular layer although accompanied by no changes in mRNA levels in the granular layer. In the cerebral cortex, a small, although statistically significant, decrease in binding was found in the deep layer (VI) (-18.3%) of aged rats, whereas no changes were found in the superficial layer (I). As in the case of the cerebellum. mRNA levels did not change in the cerebral cortex layers (II-II1 and V-VI). The different regions of the Ammon's horn of the hippocampus exhibited similar cannahinoid receptor binding levels in aged and young rats. Interestingly. mRNA levels decreased in aged rats to a small, but statistically significant. extent (CAI: -26.l0/: CA2: -21.6`,,,: CA3: -14.4"%5). This was also seen in another hippocampal structure, the dentate gyrus (---14.6%), although in this region binding levels increased in aged rats (+28.4%). Two hypothalamic structures. the arcuate nucleus and the ventromedial hypothalamic nucleus, exhibited decreased cannabinoid receptor binding in aged rats (-31.1% and -30.3`%%, respectively), but this was not seen in the medial preoptic area. This was accompanied by no changes in mRNA levels in the ventromedial hypothalamic nucleus. In the limbic structures, aged rats exhibited similar binding levels to young rats. This was seen in the nucleus accumbens. septum nuclei and basolateral amygdaloid nucleus. However, mRNA levels slightly decreased in the basolateral amygdaloid nucleus ( -13.4°,%(,). whereas they were not altered in the septum nuclei. Finally, other brain structures. such as the central gray substance and the brainstem. exhibited similar binding levels in aged and young rats. However, it is important to note that mRNA levels increased significantly (+211.20/) in the brainstem of aged rats, an area where the levels of binding and mRNA were very low in young rats. This marked increase may be related to an increase in the presence of glial elements in this region, as revealed by the increase in the immunoreactivity for glial fibrillary acidic protein observed in the brainstem of aged rats as compared to young animals. In summary, senescence was associated with changes in cannabinoid receptors in the cerebellum, the cerebral cortex, limbic and hypothalamic structures, the hippocampus and other brain regions. However. the
* Corresponding author Fax: +34 (91) 3941691;E-mail: jjfr(er 0925-4439/98/5 - see front matter rS 1998 Elscvier Science B.V. All riuhts rcser\ed.
P11: S0925-4439(98 )00042-8

206 F & rr, nderu c t at l. / Bioehinrica ct Biophv siea Act , 140' : J093; '05 '1 -l
changes observed (i) were not as marked and relevant as those early reported in extrapyramidal areas. and (ii) exhibited regional differences that might be attributed to the different roles played by these receptors in each region. Of particular relevance by their magnitude were the aging-induced decrease in binding found in the cerebellum and the hypothalamus. and the increase in mRNA levels observed in the brainstem. The latter might he related to an increase in the presence of filial cells which might contain cannabinotd receptor mRNA. ?)) 1998 Elsevier Science B.V. Drug Abuse & Addiction, Detoxification, Treatment, Opiate Withdrawal. Substance Abuse: Heroin, Cocaine, Marijuana, Crystal meth, Vicodin, OxyContin, Amphetamines, Percocet and others.
All Rights Reserved.
Keru'ordv: Cannabinoid receptor binding; Cannabinoid receptor gene; Autoradiography: In situ hybridization. Cannabinoid:. v"-Tetrahydrocannabinol: Glial fibrillary acidic protein immunohystochemistry; Glial cell: Brain: Aging
1. Introduction
A great amount of literature has allowed to demonstrate the existence of an endogenous cannabinotd system (endogenous ligands+receptor signaling pathways), which might play a role in a variety of physiological processes (for review, see [1,2]). It has been proposed. based on the distribution of cannabinoid receptor binding and mRNA levels in the brain [3-5] and on the well-known pharmacological effects of plant and synthetic cannabinoids (for review, see [6]), that the endogenous cannabinoid system would be involved in the regulation of motor behavior, cognition, learning and memory and antinociception, as well as that it might play a role in brain development [7.8].
Recent studies have demonstrated that cannabinoid receptor binding decreases in several brain regions, mainly in extrapyramidal areas, in a variety of neurodegenerative diseases, such as Huntington's chorea [9.10] or Alzheimer's disease [11]. For instance, cannabinoid receptors almost completely disappeared in the substantia nigra [9], in the globus pallidus (more markedly in the lateral part than in the medial area) [10] and, to a lesser extent, in the putamen [10] in Huntington's disease. This motor disorder is precisely characterized by a selective loss of striatal efferent neurons [12]. precisely those which contain cannabinoid receptors in the extrapyramidal circuitry [13]. Cannabinoid receptor binding also decreased in another neurodegenerative disorder, such as the Alzheimer's disease [11]. Thus, cannabinoid receptor binding decreased in the hippocampus, caudate-putamen. medial globus pallidus and substantia nigra pars reticulata, although always to a lesser extent than in Huntington's chorea, whereas no changes were seen in cannabinoid receptor mRNA expression [11].
It is important to note that in the study from
Westlake et al. [11] on the changes in cannabinotd receptors in the Alzheimer's brains, the authors believed their results to be related more to an effect of increasing age rather than selectively associated with the pathology characteristics of Alzheimer's disease. However, despite these data [11] and those obtained in other neurodegenerative diseases [9,10], there is scarce evidence in regard to potential changes of these receptors during normal senescence. It would be attractive if the synthesis, binding and/or functionality of cannabinoid receptors in the brain might be altered in aged reported for the receptors of other neurotransmitters [14-16]. thus suggesting their possible use as a molecular marker in normal and pathological aging. In this sense, it is interesting to note that the impairment of functions associated with marihuana consumption, in terms of psychomotor performance, immediate memory. motor coordination and others, declines with age [17]. It is likely that these effects associated with marihuana exposure might be undoubtedly caused by the activation of cannabinoid receptors located in the brain structures related to those neurobiological processes, such as the basal ganglia, the hippocampus. the cerebellum, the limbic nuclei and others. which present measurable binding levels for these receptors [3.4].
In this sense. Belue et al. [18] have recently reported that cannabinoid receptor binding did not change during the normal aging process in rats.. but this study was quite limited since it was performed in whole brain and using membrane binding techniques. Previously, Mailleux and Vanderhaeghen [19] reported a reduction in the expression of cannabinoid receptors and mRNA levels in the striatum of aged rats, although these authors did not examine other brain areas. In a recent study, our group has found that cannabinoid receptor binding and gene expression, measured by autoradiography and in situ hybridization, respectively, markedly decreased in ex-
F. Berrendero et al. l Biochinaica et Biophvsica Acta 1407 (1998) 205-214 207
trapyramidal structures of aged rats [20]. We have found a selective aging-induced reduction in cannabinoid receptors located in striatonigral and striatoentopeduncular neurons, but a maintenance in striatopallidal neurons [20], all accounting for the progressive motor impairment characteristic of normal senescence. However, in our previous study [20], only extrapyramidal areas were examined and nothing was reported about other regions that are relevant from the view of an important presence of cam nabinoid receptors. such as the hippocampus. cerebellum, limbic structures. cerebral cortex and others. The present study was designed to determine the possible existence of aging-induced changes in cannabinoid receptor binding and gene expression in brain regions other than extrapyramidal structures. To this end, we analyzed, using autoradiography with [3H]WIN 55,212-2. the specific binding of cannabinoid receptors in slide-mounted brain sections obtained from young (3 month old) and aged (> 2 year old) rats. This experiment was completed with the analysis of cannabinoid receptor mRNA levels, using in situ hybridization, in slide-mounted brain sections also obtained from young and aged rats. Finally, we also analyzed glial fibrillary acidic protein (GFAP) immunoreactivity in brain sections of young and aged rats that contain the brainstem. The specific objective of this study was to demonstrate that the high presence of cannabinoid receptor mRNA levels in this area in aged rats might be related to an increase in the presence of glial cells in senescence.
2. Materials and methods
2.1. Animals, sampling and tissue preparation
Two age groups of male Wistar rats were used in this study: young rats (3 months old) and aged rats ( > 2 years old). They were housed in a room with controlled photoperiod (08.00-20.00 h light) and temperature (23±1°C). They had free access to standard food and water. At the two ages described above, young and aged rats were used for two different experiments. In the first experiment, animals were sacrificed by rapid decapitation and their brains quickly and carefully removed and rapidly frozen
by immersion in 2-methylbutane cold in dry ice (T= -30°C). All samples were stored at -70°C until processed. Coronal sections 20 µm thick were cut in a cryostat, according to the atlas of Paxinos and Watson [21]. Sections were thaw-mounted onto RNase-free gelatin/chrome alum coated slides and dried briefly at 30°C and stored at -80°C until use. For the identification of the different brain nuclei, adjacent sections to those used for autoradiographic analysis were stained with cresyl violet and analyzed according to the atlas of Paxinos and Watson [21]. Sections from six different animals per group were used for each of the two autoradiographic analyses. In the second experiment. animals were anesthetized with ether and perfused transcardially with 2.5% paraformaldehyde in phosphate buffer (pH 7.2). The brains were dissected and postfixed in the same fixative for 4 h at 4°C. Tissue blocks were dehydrated and embedded in paraffin. Adjacent sections of 8 µm thick were processed for immunodetection of GFAP.
2.2. AutoradiographY of cannabinoid receptor binding
The protocol used is basically the method described by Jansen et al. [22] with modifications. Briefly, slide-mounted brain sections were preincubated for 20 30°C. in a buffer containing 20 mM HEPES with 0.5 bovine serum albumin (fatty acid-free). pH 7.0. Slides were then incubated for 80 min, at 30°C, with 1 nM [3H]WIN 55.212-2 (Du Pont NEN, Itisa. Madrid, Spain) prepared in the same buffer, in the absence or the presence of 10 µM unlabeled WIN 55.212-2 (RBI. Natick. MA, USA) to determine total and non-specific binding, respectively. Following this incubation, slides were washed in buffer four times (10 min each) at room temperature. dipped in ice-cold distilled water and then dried under a stream of cool dried air. Autoradiograms were generated by apposing the labeled tissues. together with autoradiographic standards ([-'H]-micro-scales, Amersham Iberica, Madrid, Spain), to tritium-sensitive film (['HI-Hyperfilm, Amersham Iberica) for a period of 3-4 weeks, and developed (D-19, Kodak) for 4 min at 20°C. Developed films were analyzed and quantitated in a computer-assisted videodensitometer (Image Quant 3.3. Molecular Dynamics) using the standard curve gen-
208
F. Berrendero et al. I Bioch/ pica et Biopln•sica rl cta 1407 ! 1498 i 205-214
crated from ['H] standards. After logarithmic transformation. all data were best fitted to a linear equa
Analysis of cannabinoid receptor niRNA levels biin situ hybridization
In situ hybridization was carried out according to the procedure previously described by Rubino et al. [23] with slight modifications. Briefly, sections were fixed in 4°/) formaldehyde for 5 min and, after rinsing twice in phosphate buffer saline, acetylated by incubation in 0.25% acetic anhydride, prepared in 0.1 M triethanolamine/0.15 M sodium chloride (pH 8.0), for 10 min. Sections were rinsed in 0.3 M sodium chloride/0.03 M sodium citrate (pH 7.0), dehydrated and delipidated by ethanol/chloroform series. A mixture (1:1:1) of the three 48-mer oligonucleotide probes complementary to bases 4-51, 349-396 and 952-999 of the rat cannabinoid receptor eDNA (Du Pont. Itisa. the specificity of the probes used
was assessed by Northern blot analysis, data not shown) was 3'-end labeled with [3aS]dATP (Amersham Iberica) using terminal deoxynucleot.idyl transferase (Boehringer Mannheim.. Barcelona. Spain). Sections were then hybridized with "S-labeled oligonucleotide probes (2.5 X 10" dpm per section), washed and exposed to X-ray film ([3max, Amersham Iberica') for 10 days, and developed (D-19, Kodak) for 6 min at 20°C. The intensity of the hybridization signal was assessed by measuring the grey levels in the auto radiographic films with a computer-assisted videodensitometer (Image Quant 3.3, Molecular Dynamics). Additional brain sections were co-hybridized with a 100-fold excess of cold probe or with RNAse to assert the specificity of the signal. As expected, no hybridization signal was detected in these sections (data not shown).
2.4. Inmmunohistoc% emical analysis of GFAP
Sections were deparaffined and treated with non
Table I
Cannabinoid receptor binding (fmol/mg tissue) measured by autoradiography in several brain areas of young (3 months) and aged
|
( > 2 years) male rats |
|||
|
Brain region |
Cannabinoid receptor binding (fmollmg tissue) |
||
|
Young rats |
Aged rats |
Change (%) |
|
|
Hippocampus Amman's horn CAI |
104.7 ± 3.6 |
95.8 ± 8.9 |
-8.5 |
|
CA2 |
98.1 t 4.2 |
95.4 ± 6.5 |
-2.8 |
|
CA3 |
68.3 t 3.2 |
67.8 ± 6.0 |
-0.7 |
|
Dentate gyrus |
72.1 ± 4.4 |
92.6±7.1* |
+28.4 |
|
Cerebral cortex Superficial laver (I) |
78.7±6.9 |
77.0 t 5.1 |
-2.2 |
|
Deep layer (VI) |
70.1 +3 8 |
57_3±4.9* |
-18.3 |
|
Cerebc'ihim Molecular later |
185.7 ± 11.7 |
124.1 ± 15.7** |
|
|
Limbic nuclei Nucleus accumbens |
53.1 ±4.5 |
55.6 ± 6.8 |
+4.7 |
|
Septum nuclei |
53.4+8.1 |
51.1 ± 5.7 |
-4.3 |
|
Basolateral amv_ gdaloid nuclei |
29.9 ± 3.3 |
29.8 ± 3.7 |
-0.3 |
|
Hypothalamus Arcuate nucleus |
19.6 ± 2.0 |
13.5 ± 2.5* |
-31.1 |
|
Ventromedial hypothalamic nucleus |
21.1 t 2.5 |
14.7 t 1.3* |
-30.3 |
|
Medial preoptic area |
17.0± 1.4 |
14.3 ± 3.5 |
-15.9 |
|
Miscellaneous Central gray substance |
19.6 t 2_6 |
17.5 ± 2.2 |
-10.7 |
tion--3
Values are means ±S.E.M. of six animals per group. Data were analyzed by Student's t-test. *P < 0.05.
**P < 0.005.
F Berrendero et al. /Biochimica et Biophrsica Acta 140' (1998) 205-214
209
immune serum diluted 1/30 in Tris buffer (pH 7.6) for 30 min. Slides were incubated in polyclonal GFAP antiserum (Dakopatts), diluted 1/1000 in Tris buffer for 24 h at 4°C. Following three 5 min washes in Tris buffer, sections were incubated in swine anti-rabbit IgG, diluted 1150 for 1 h at 20°C, washed in Tris buffer and incubated in rabbit PAP complex, diluted 1/200, for l h at 20°C. Thereafter, sections were rinsed twice in Tris buffer and the peroxidase activity was demonstrated with 0.03% DAB (Sigma, Madrid. Spain) in 0.05 M Tris buffer with 0.005'i,, H,O,. washed in distilled water, dehydrated in graded concentrations of ethanol and mounted in DePeX. Some sections were incubated with preimmune swine serum at 1/30 dilution as the primary antiserum. These control sections showed no immunoreactive product.
2.5. Statistics
Autoradiographic data of cannabinoid receptor binding and mRNA levels in young and aged rats were assessed by Student's t-test.
3.- Results
3.1. Cerebellum
Aged rats exhibited a marked decrease in cannabinoid receptor binding in the molecular layer (-33.3%, see Table 1), although not accompanied by changes in mRNA levels in the granular layer (Table 2). See representative autoradiograms for both binding and mRNA levels in Figs. 1 and 2.
3.2. Cerebral cortex
A small, although statistically significant. decrease in cannabinoid receptor binding was found in the deep layer (VI) of the cerebral cortex (-18.3°/0 of aged rats. whereas no changes were found in the superficial layer (I) (Table 1). As in the case of the cerebellum, cannabinoid receptor mRNA levels did not change in the cerebral cortex layers (II-I11 and V-VI) (Table 2). See representative autoradiograms for both binding and mRNA levels in Figs. 1 and 2.
|
Table 2 Cannabinoid receptor mRNA levels (expressed as arbitrary units of optical density) measured by in situ hybridization in several brain areas of young (3 months) and aged ( > 2 years) male rats |
|||
|
Brain region |
Optical density (arbitrary units) |
||
|
Young rats |
Aged rats |
Change (`5,) |
|
|
Hippocanrpus Ammon's horn CA] |
0.337 ± 0.034 |
0.249 ± 0.013* |
-26.1 |
|
CA2 |
0.399 ± 0.040 |
0.313 t 0.018* |
-21.6 |
|
CA3 |
0.416 10.019 |
0.356±0.019* |
-14.4 |
|
Dentate gyrus |
0.411 ± 0.010 |
0.351 ±0.015** |
-14.6 |
|
Cerebral cortex Layers 11-111 |
0.228 ± 0.017 |
0.255±0.016 |
+11_8 |
|
Layers V-VI |
0.235 ± 0.013 |
0.239 ±0.008 |
+1.7 |
|
Cerebellum Granular layer |
0.884±0,047 |
0.960 ± 0.080 |
+8.6 |
|
Limbic nuclei Septum nuclei |
0.156 ± 0.014 |
0.179 ± 0.005 |
+14.7 |
|
Basolateral amygdaloid nuclei |
0.352±0.019 |
0_305 ± 0.013* |
-1 3.4 |
|
Hrpothalanmus Ventromedial hypothalamic nucleus |
0.333 ± 0.025 |
0.322 ±0.022 |
-33 |
|
Miscellaneous Brainstem |
0.178 ± 0.026 |
0.554 ± 0.140** |
+2_.2 |
11
Values are expressed as means +S_E.M. of six animals per group. Data were analyzed by Students i-test. * P < 0.05.
210
F Bcrr•etudero et al. /Bioihirrrica et Bropln'sica Actct 140? (1 VIM"05- 214
Young rats
Aged rats
Nonspecific binding
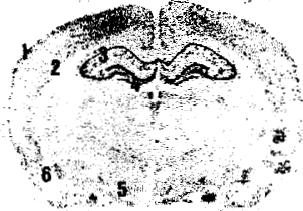
Fig. 1. Representative autoradiograms (5 x) for cannabinoid receptor binding at the level of plates 28 and 68 according to the atlas of Paxinos and Watson [21]. obtained from slide-mounted brain sections of young (3 months; left panels) and aged (>2 years; middle panels) male rats. Right panels correspond to non-specific binding in the sections of young rats (similar autoradiograms were obtained for the non-specific binding in aged rats). Autoradiograms were processed according to the conditions described in Section 2. 1. superficial layers of the cerebral cortex; 2. deep layers of the cerebral cortex: 3. Ammon's horn: 4. dentate gyrus, 5. molecular layer of the cerebellum; 6, brainstem.
3.3. Hippocampus
The different regions of the Ammon's horn of the hippocampus exhibited similar cannabinoid receptor binding levels in aged and young rats (Table 1). Interestingly, cannabinoid receptor mRNA levels decreased in aged rats to a small, but statistically significant, extent (CAI: -26.1%; CA2: -21.6°/x; CA3: -14.4% see Table 2). This was also seen in another hippocampal structure, the dentate gyrus (-14.6%: see Table 2). although in this region, cannabinoid receptor binding slightly increased in aged rats (+28.4°%%,:: see Table 1). See representative autoradiograms for both binding and mRNA levels in Figs. 1 and 2.
3.4. Hypothalamus
Two hypothalamic structures, the arcuate nucleus and the ventromedial hypothalamic nucleus, exhibited decreased cannabinoid receptor binding in aged rats (-31.1% and -30.3%, respectively; see Table 1), but this was not seen in the medial preoptic area (Table 1). This was not accompanied by changes in mRNA levels in the ventromedial hypothalamic nu
cleus (Table 2). See representative autoradiograms for both binding and mRNA levels in Figs. 1 and 2.
3.5. Limbic structures
Aged rats exhibited similar cannabinoid receptor binding levels as young rats (Table 1). This was seen in the nucleus accumbens as well as in the basolateral amygdaloid nucleus and septum nuclei. However, mRNA levels slightly decreased in the basolateral amygdaloid nucleus (-13.4%). whereas they were not altered in the septum nuclei (Table 2). See representative autoradiograms for both binding and mRNA levels in Figs. 1 and 2.
3.6. Miscellaneous
We also analyzed other brain structures, all containing very low levels of cannabinoid receptor binding and mRNA, such as the central gray substance and the brainstem. Cannabinoid receptor binding levels were similar in the central gray substance of aged and young rats (Table 1), whereas mRNA levels were practically non-detectable. Contrarily, cannabinoid receptor binding levels were practically similar





F Berrendern et al. l Biochimica et Biopln,sica Acta 1407 (1998) 205-214 211
Young rats
Aged rats
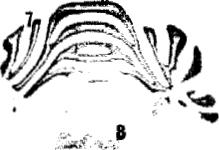
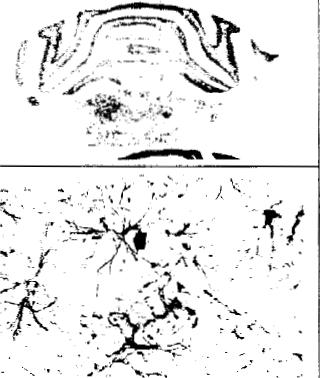
Fig. 2. Representative autoradiograms for cannabinoid receptor mRNA levels (top and middle panels: 5 x) and photomicrographs for GFAP immunoreactivity (bottom panels: 450x) at the level of plates 28 and 68 according to the atlos of Paxinos and Watson [21], obtained from slide-mounted brain sections of young (3 months: left panels) and aged ( > 2 years: right panels) male rats. Autoradiograms and photomicrographs were processed according to the conditions described in Section 2. 1 _ superficial layers of the cerebral cortex; 2, deep lavers of the cerebral cortex: 3. Ammon's horn:; 4. dentate gyrus: 5, ventromedial hypothalamic nucleus; 6. basolateral amygdaloid nucleus: 7, granular layer of the cerebellum: 8, brainstem: arrow. GFAP-positive cells.
to non-specific binding in the brainstem of both young and aged rats, whereas mRNA levels were detectable and increased significantly (+211 2%) in aged rats (Table 2). See representative autoradiograms for both binding and mRNA levels in Figs. 1 and 2. This increase runs in parallel to an increase in GFAP immunoreactivitv observed in the brainstem of aged rats as compared to young animals (see Fig. 2). This might indicate that the high levels for cannabinoid receptor mRNA in this area might be located in glial cells, particularly astrocytes.
4. Discussion
In our previous study [20], we have seen that aging
produces a marked drop in cannabinoid receptor binding and mRNA expression in extrapyramidal structures. This might be related to the decrease in these receptors observed in several neurodegenerative diseases affecting the basal ganglia such as the Huntington's chorea [9,10]. In the same line. Westlake et al. [11] analyzed the changes in cannabinoid receptors in Alzheimer's brains and concluded that these changes were mainly observed in the basal ganglia and were related more to an effect of increasing age rather than selectively associated with the pathology characteristics of Alzheimer's disease. In the present study, we have seen that the changes found in the basal ganglia of aged rats [20] were also observed in brain regions other than extrapyramidal areas. although these changes were mostly less


212
F. Brrrcnzlero et al.IBincdinric'a ct Biop{cr.rica Ac1u 1--1117 11998, 205 ?14
marked. Moreover, the changes observed showed regional differences that possibly indicate a selective alteration of cannabinoid receptor-containing neurons in the different areas.
There was a marked decrease in cannabinoid receptor binding in the molecular layer of the cerebellum of aged rats. However, as a difference versus extrapyramidal structures [20], the levels of gene transcripts were not altered in the granular layer. the layer where most of the cell bodies of cannabinoid receptor-containing neurons in the cerebellum are clustered [5]. This decrease, only observed in receptor binding, might indicate that axonal terminals in the molecular layer, those containing cannabinoid receptors, may be affected earlier or more severely than their cell bodies or dendrites in the granular layer, as suggested by Richfield and Herkenham [10] in the globus pallidus of Huntington's brains. Alternatively. it might be considered that this difference, decreases in binding and no changes in gene expression, might reflect an impairment preferentially associated with deficient production, processing or transport of cannabinoid receptors to axonal terminals. An unlikely possibility would be that the decrease in binding might reflect the aging-induced death of neurons containing these receptors, because, in that case, a similar decrease in gene transcripts should be expected.
Decreases in binding and no changes in gene expression were also seen in other structures, and similar explanations of the possible mechanisms involved may be applied to these structures. Among them, we could mention the deep layers of the cerebral cortex and the arcuate and ventromedial nuclei of the hypothalamus. The changes in the cerebral cortex were very small, although statistically significant, and only observed in the deep layers. likely indicating a probably limited physiological relevance. However, the changes in binding observed in the two hypothalamic nuclei were more marked (more than 30°h decrease), although, as in the case of the cerebellum, they were not accompanied by changes in mRNA levels in one of these two nuclei, the ventromedial nucleus, where cell bodies of cannabinoid receptor-containing hypothalamic neurons have been reported to be located [5].
In other structures, however, the changes observed during senescence mainly affected gene expression,
but not binding levels. This was the case in the different subfields of the Ammon's horn of the hippocampus, an area where cannabinoid receptors have been reported to be located on both intrinsic and extrinsic neurons [5]. Thus, mRNA levels slightly decreased, but in a statistical significant manner.. in the CAI, CA2 and CA3 layers, with no changes in binding. In our opinion, this might be interpreted as an aginginduced primary reduction in receptor synthesis due to a decrease in gene expression and/or in mRNA stability, but the small magnitude of the decrease appears to be not enough to originate a parallel loss in binding capability. It would be possible that the aging-induced decrease in gene expression, although not affecting the basal levels of receptor binding, might originate a plausible deficit of these neurons to respond to a variety of circumstances that demand the de novo synthesis of receptors. A similar kind of aging-induced alterations, decreases in gene expression and no changes in binding, was seen in the basolateral amygdaloid nucleus, and similar explanations of possible mechanisms and physiological relevance may be applied to this structure. In another hippocampal structure, the dentate gyrus, mRNA levels also decreased in aged rats, but in this case the levels of specific binding increased, which might indicate the existence of a differential effect of aging on cannabinoid receptor-containing neurons in this region: decreased receptor synthesis, but increased receptor binding capability. Further research should provide additional evidence on this paradoxical result.
Finally, it is noteworthy to mention the case of the brainstem. This area contains a relatively low population of cannabinoid receptors. The levels of binding measured by other authors were very low [3-5], and in our present study practically non-existent since they were scarcely different from background. However, the levels of gene transcripts, which have been previously reported to be also very low in adult animals [5], markedly increased (more than 3-fold higher) in aged rats. There was no specific location of this increase in specific areas of the brainstem, although it might be appreciated higher grey levels in the areas of raphe nuclei and reticulo-tegmental formation, where the cell bodies of monoaminergic neurons are located. These- -neurons, which do not contain cannabinoid receptors in adult individuals, project
F Berrendero et al. I Biochimka el Biophysica Acla 1407 (1998,; 205-214
213
to most of the forebrain structures. However, we have found no increases in cannabinoid receptor binding in the forebrain structures, which precludes the possibility that cannabinoid receptors might be significantly expressed during aging in these neurons. An attractive possibility would be that the marked increase found in mRNA levels in the brainstem of aged rats might be related to the expression of cannabinoid receptors in non-neuronal elements, such as glial cells. This would be similar to what occurs in the developing brain where transient expression of these receptors in filial cells has been proposed [8.25-27]. It has been reported that the neuronal death that appears during normal and pathological aging is also associated with the appearance of glial elements that replace dead neurons [24]. In support of this hypothesis, we can argue that the magnitude of the increase in mRNA levels in the brainstem found in this study varied significantly in the aged rat group, with most of the animals exhibiting higher levels, but a few having levels similar to young rats. It would be possible that the increase in mRNA levels might run in parallel to the degree of neuronal death and glial cell replacement. In the present study, we have tried to validate this hypothesis by examining the immunoreactivity for GFAP, which is a marker of the same regions of the brainstem where cannabinoid receptor rnRNA levels increased in aged rats. Our results confirmed that an increased immunoreactivity for GFAP exists in the brainstem of aged rats as compared to young animals. This increase was originated by both an increased number of GFAP-positive neurons and increased immunoreactivity for GFAP in each individual cell.
In summary, senescence was associated with changes in cannabinoid receptors in the cerebellum, the cerebral cortex, limbic and hypothalamic structures, the hippocampus and other brain regions. However, the changes observed (i) were not as marked and relevant as those early reported in extrapyramidal areas [20], and (ii) exhibited regional differences that might be attributed to the different roles played by these receptors in each region. Of particular relevance by their magnitude were the aging-induced decrease in cannabinoid receptor binding found in the cerebellum and the hypothalamus, and, in particular, the increase in mRNA levels ob
served in the brainstem. It appears feasible that the increase observed in the brainstem of aged rats might reflect the expression of this gene in glial cells, whose number increased significantly in this region in senescence.
Acknowledgements
This work has been supported by grants from CICYT (PM96-0049) and UCM (PR294/95-6189). J. Romero is a post-doctoral fellow of the "Comunidad Autonoma de Madrid". The technical assistance of Ana Jurado is gratefully acknowledged.
References
[1] R. Mechoulam. L. Hanus. B.R. Martin, Search for endogenous ligands of the cannabinoid receptors. Biochem. Pharmacol. 48 (1994) 1537-1544.
[2] R.G. Pcrtwee, Pharmacological. physiological and clinical implications of the discovery of cannabinoid receptors: an overview, in: R.G. Pertwee (Ed.), Cannabinoid Receptors. Academic Press, London. 1995- pp- 1-34.
[31 M. Herkenham, A.B. Lynn, M.D. Little, M.R. Johnson.. L.S. Melvin, D.R. de Costa. K.C. Rice, Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA 87 (1990) 1932-1936.
[4] M. Herkenham, A.B. Lynn. M.R. Johnson. L.S. Melvin, B.R. de Costa. K. C. Rice. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 11 (1991) 563-593
[5] P. Mailleux, J.J. Vanderhaeghen, Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautoaraphy and in situ hybridization histochemistry, Neuroscience 48 (1992) 655-668.
[6] A.C. Howlett, Pharmacology of cannabinoid receptors, Annu. Rev. Pharmacol. Toxicol. 35 (1995) 607-634.
[7] J.J.Fern andez-Ruin, A. Bonnin, M. Cebeira, J.A. Ramos. Ontogenic and adult changes in the activity of hypothalamic and extrahypothalamic dopaminergic neurons after perinatal cannabinoid exposure. in_ T. Palomo. T. Archer (Eds.). Strategies for Studying Brain Disorders. Farrand Press, London, 1994. pp. 357-390.
[81 J. Romero, E. Garcia-Palomero, F. Berrendero, ..4 Ramos. J.J. Fernandez-Ruiz. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse 26 (1997) 317 323.
[9] M. Glass. R.L.M. Fault. M. Dragunow, Loss of cannabinoid receptors in the substantia nigra in Huntington's disease, Neuroscience 56 (1993) 523-5_27.
214
F. Berrendero et ul. /Biochirttiea tit Bitrpltrsicu ,4cta 1407 (199Ri 205-_14
[10] E.K. Richfield. M. Herkenham. Selective vulnerability in Huntington's disease: preferential loss of cannabinoid receptors in lateral globus pallidus, Ann. Neurol. 36 (1994) 577584.
[11] T.M. Westlake. A.C. Howlett. T.I. Bonner. L.A. Matsuda, M. Herkenham, Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer's brains. Neuroscience 63 (1994) 637-652.
[12] for degenerative and regenerative changes in neostriatal spiny neurons in Huntington's disease. Science 227 (1985) 770--773.
[13] M. Herkenham, A.B. Lynn. B.R. de Costa, E.K. Richfield, Neuronal localization of cannabinoid receptors in the basal ganglia of the rat, Brain Res. 547 (1991) 267-274.
[14] L.F. Agnati, K. Fuxe, on aging processes.. Acta Physiol. Scand. Suppl. 532 (1984) 45-61
[15] J.J. Fernandez-Ruiz, R. de Miguel, M.L. Hernandez, M. Cebeira, J A Ramos, Comparisons between brain dopaminergic neurons of juvenile and aged rats: sex-related differences, Mech- Ageing Dev. 63 (1992) 45-55.
[16] G.S. Roth, J.A. Joseph. Age-related changes in transcriptional and posttranscriptional regulation of the dopaminergic system. Life Sci. 55 (1994) 2031-2035.
[17] M.I. Soueif. Differential association between chronic cannabis use and brain function deficits, Ann. NY Acad. Sci. 282 (1976) 323-343.
[18] R.C. Belue, A.C. Howlett. T.M. Westlake, E.D. Hutchings.. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats, Neurotoxicol. Teratol. 7 (1995) 25-30.
[19] P. Mailleux. J.J. Vanderhaeghen. Age-related loss of cannabinoid receptor binding sites and mRNA in the rat striatum. Neurosci. Lett. 147 (1992) 179-181.
[20] J. Romero, F. Berrendero. I.. Garcia-Gil, P. de la indez-Ruiz, Loss of cannabinoid receptor binding and mRNA levels and cannabinoid agonist-stimulated [`'S]GTP fS binding in the basal ganglia of aged rats, Neuroscience 84 (1998) 1075-1083,
[21] G. Paxinos. C. Watson. The Rat Brain in Stereotaxic Coordinates. Academic Press. London, 1986.
[22] of cannabinoid receptors in rat brain determined with aminoalkylindoles, Brain Res. 575 (1992) 93-102.
[23] T. Rubino. P. Massi.. G. Patrini, I. Venier, G. Giagnoni. D. Parolaro. Chronic CP-55.940 alters cannabinoid receptor mRNA in the rat brain: an in situ hybridization study, Neuroreport 5 (1994) 2493-2496
[241 R.H. Myers. J.P. Vonsattel. P.A. Paskevich, D.K. Kiely. T.J. Stevens, L.A. Cupples, E.P. Richardson Jr., E.D. Bird, Decreased neuronal and increased oligodendroglial densities in Huntington's disease caudate nucleus. J. Neuropathol. Exp. Neurol. 50 (1991) 729-742.
[25] F. Berrendero, I.. Garcia-Gil, M.L. Hernandez.. J. Romero. M. Cebeira, R de Miguel, of mRNA expression, binding and activation of signal transduction mechanisms for the cannabinoid receptor in the rat brain during fetal development, Development (1998) in press.
[26] M. Bouaboula, B. Bourrie. M. Rinaldi-Carmona, D. Shire, G. Le Fur. P. Casellas, Stimulation of cannabinoid receptor CBI induces krox-24 expression in human astrocytoma cells. J. Biol. Chem. 270 (1995) 13973-13980.
[27] A.C. Shivachar, B.R. Martin, E.F. Ellis, Anandamide- and A9-tetrahydrocannabinol-evoked arachidonic acid mobilization and blockade by SR 141716A [N-(piperidin-1-yl)-5-(4chlorophen_vl)-1-(2,4-dichlorophenyl)-4-methyl-1 H-pyrazole3-carboximide hydrochloride], Biochem. Pharmacol. 51 (1996) 669-676.
| Index |
| Index |
Last Updated (Monday, 20 December 2010 15:14)












