Cannabinoid Dependence in Mice
Drug Abuse
Cannabinoid Dependence in Mice 1151
investigators found withdrawal signs only when chronically treated Q9-THC rats were challenged with a high dose of naloxone (Hirschhorn and Rosecrans, 1974). Others reported an abstinent syndrome that occurred only after the administration of neurotransmitter reuptake inhibitors, either clomipramine, imipramine or fluoxetine, the day after cessation of chronic Q9-THC treatment (Taylor and Fennessy, 1982; Verberne et al., 1980). It is unclear whether these behavioral effects were due to withdrawal or a drug interaction.
An effective means of demonstrating dependence is to precipitate physical withdrawal by challenging chronically treated animals with an appropriate antagonist. Discovery of a long-awaited cannabinoid antagonist has made it possible to assess dependence by conducting precipitated withdrawal studies. The novel competitive cannabinoid antagonist SR141716A binds with high affinity to the central CB1 cannabinoid receptor and effectively antagonizes a variety of cannabinoid effects in rodents (Compton et al., 1996; RinaldiCarmona et al., 1994, 1996). Recently, it was shown that SR141716A was capable of precipitating a withdrawal syndrome in rats chronically treated with Q9-THC (Aceto et al., 1995, 1996; Tsou et al., 1995). Upon termination of Q9-THC treatment and immediate administration of SR141716A, a profound abstinence syndrome was evident by the appearance of overt behavioral signs in these chronic drug-treated rats. The withdrawal signs included wet-dog shakes, involuntary paw tremors, ptosis, tongue rolling, retropulsion, head shakes and facial rubbing. These studies were the first to demonstrate unequivocally that chronic cannabinoid treatment resulted in a physical withdrawal syndrome in rats.
An important question is whether precipitated cannabinoid withdrawal occurs in other species. Our knowledge regarding the pharmacological actions of cannabinoids has been derived from numerous animal species. For example, pharmacological tolerance and cross-tolerance studies with Q9-THC, CP-55,940, WIN 55-212 and anandamide have been established using mouse behavioral models and smooth muscle preparations (Fan et al., 1994; Pertwee, 1993; Pertwee et al., 1992, 1995). In addition, localization of the cannabinoid receptor and second messenger systems and changes in receptor number and mRNA levels for the cannabinoid receptor have been investigated in mice (Abood et al., 1993; Fan et al., 1996; Herkenham et al., 1991). In short, development of cannabinoid dependence in a second species, such as the mouse, would provide further credence for this phenomenon that has been characterized in the rat. Additionally, the development of a mouse model of dependence would be beneficial since so much is known regarding the cannabinoid system in this species.
Materials and Methods
Animals. Male ICR mice (Harlan Laboratories, Dublin, VA) weighing 20 to 27 g and housed six mice per cage were used in all experiments. Mice were maintained on a 14:10 hr light:dark cycle with water and food ad libitum.
Drug preparation. Q9-THC was provided by the National Institute on Drug Abuse, Rockville, MD, and SR141716A was generously donated by Pfizer Central Research (Groton, CT). All drugs were dissolved in a 1:1:18 solution of ethanol, emulphor and 0.9% saline. Emulphor (EL-620, a polyoxyethylated vegetable oil, GAF Corporation, Linden, NJ) is currently available as Alkmulphor. All s.c. and i.p. injections were administered at a volume of 0.1 ml/10 g of body
weight. On test day mice were acclimated in the laboratory overnight without interruption of food and water.
Tolerance development and antagonist challenge. For six days either Q9-THC (10 mg/kg) or vehicle (1:1:18) was given s.c. twice a day, once between 09.00-11.00 hr and again between 21.00-23.00 hr. This regimen has been shown to produce tolerance to the antinociceptive, locomotor, hypothermic and cataleptic effects of Q9-THC (Abood et al., 1993; Fan et al., 1994). Body weights were recorded and used as an indicator of toxicity. On day 7, the test day, mice received an acute i.p. challenge with either SR141716A or vehicle 4 hr after their last chronic Q9-THC treatment. For studying the time course of dependence development, the same protocol was used except in addition to a 7-day dosing regimen, separate groups of mice were also dosed for either 1, 2, 3 or 14 days. For example, mice received Q9-THC (10 mg/kg) or vehicle in the morning followed by SR141716A or vehicle 4 hr later for the day 1 time point.
Behavioral evaluation. Immediately after either SR141716A or vehicle challenge, mice were observed for 30 min (except where otherwise noted) in clear activity cages for typical withdrawal behaviors and any unique behavior. These typical behaviors included head shakes (turning or twisting head side to side), paw tremors, retropulsion (more than three steps backward), writhing, scratching, rubbing, grooming, piloerection, penile erection and Straub tail. Paw tremors were rapid lateral movements of the paws that typically lasted several seconds and were episodic which allowed for quantitation. A grooming episode was typically characterized by the licking of paws and body and by rubbing paws over nose, head and ears.
Different mice were used for each test and time point; and there were at least six mice per group. Experimenters were blind to the drug conditions in all experiments. All studies were approved by the Institutional Animal Care and Use Committee.
Statistics. Data were analyzed by ANOVA at P < .05. Bonferroni post hoc analyses (comparison with vehicle) were used when appropriate. For dose-response curves, Emax values were calculated with ALLFIT with the minimum being constrained to vehicle-vehicle values. A modification of the method of Tallarida and Murray (1987) was used to calculate ED50 values and 95% C.L. ED50 values were not calculated if one-way ANOVA was not significant at P < .05. Each point represents at least six mice per dose and dose-effect curves consist of at least three doses.
Results
Before designing a model of precipitated withdrawal in THC-dependent mice, it was essential to characterize the pharmacological effects of the antagonist alone. Doses of 10 and 30 mg/kg of SR141716A were administered i.p.; and
experimenters blind to the drug conditions observed the mice for 30 min for behaviors similar to those reported in rats undergoing precipitated withdrawal, as well as any unique behaviors (fig. 1). The most prominent behavioral signs tal
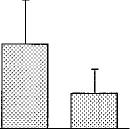
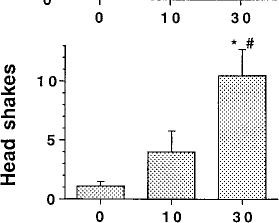
lied were paw tremors, head shakes and scratching. There were no significant differences in the number of paw tremors between vehicle and either dose of SR141716A (P = .15). On the contrary, the highest dose of SR141716A, 30 mg/kg, elic
ited a significant number of head shakes when compared with vehicle and a dose of 10 mg/kg of SR141716A. SR141716A alone elicited a marked increase in scratching in naive mice (F = 14.329, P < .05). Both doses of SR141716A
were significantly different from vehicle.
Because the lower dose of SR141716A failed to elicit a significant number of paw tremors and head shakes during the 30-min period after injection, a subsequent experiment
was conducted in which mice were challenged with SR141716A (10 mg/kg) and observed for 1 hr. Several behavioral signs were recorded at 15-min intervals to establish the
Vol. 285
U) L
0
E
0
L M+
co a
0 10 30
SR141716A (mg/kg)
Fig. 1. SR141716A-induced paw tremors, head shakes and scratching in naive mice. Naive mice received an acute i.p. administration of either SR141716A (10 or 30 mg/kg) or vehicle (1:1:18). Immediately after drug administration, the number of paw tremors (A), head shakes (B) and scratching (C) were recorded for 30 min. Results are presented as mean ± S.E.M. for 6 to 12 mice. *P < .05, SR141716A is significantly different than vehicle. #P < .05, SR141716A (30 mg/kg) is significantly different than SR141716A (10 mg/kg).
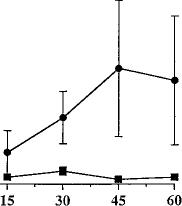
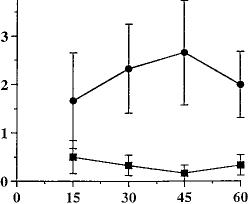
time course of the acute effects of SR141716A (fig. 2). The data were analyzed by two-way ANOVA for repeated measures. There was no drug effect (P = .71) or time effect (P = .4936) for paw tremors. SR141716A did, however, elicit a significant drug effect for head shakes (F = 5.37, P < .05) but failed to show a time effect (P = .87) or interaction (P = .51). Because the behavior in mice treated acutely with SR141716A or vehicle did not differ at either 15 or 30 min, or when the data for these two time intervals were collapsed, the dependence experiments were conducted for 30 min immediately after the acute challenge with either of SR141716A or vehicle.
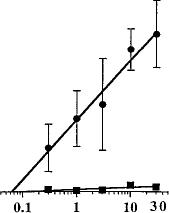
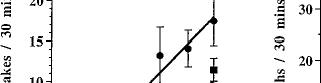
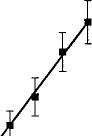
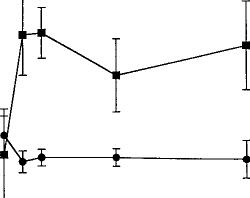
SR141716A markedly increased the number of paw tremors in a dose-dependent fashion in mice chronically treated with O9-THC (fig. 3A). As the dose of SR141716A increased, the number of paw tremors in O9-THC chronically treated mice increased with an ED50 value (95% C.L.) of 4.6 mg/kg (2.5-8.2). Mice treated chronically with vehicle and challenged with SR141716A at doses up to 30 mg/kg did not differ significantly from vehicle-vehicle treated mice with respect to paw tremors. Therefore, an ED50 value for mice chronically treated with vehicle could not be calculated. SR141716A also dose-dependently increased the number of head shakes in mice, but did so for both groups (fig. 3B). Using ALLFIT analysis and a modification of the method of Tallarida and Murray, the ED50 value (95% C.L.) for O9-THC chronically treated mice was 1.3 mg/kg (0.6-2.7). The ED50 value for
15
0
0
4
Time (minx)
Fig. 2. Time course of SR141716A effects on behavior in naive mice. Naive mice received an acute administration of either SR141716A (10 mg/kg, i.p.) (F) or vehicle (1:1:18, i.p.) (f). Behavior was recorded for 60
min in 15-min intervals immediately after drug administration. Results were analyzed by ANOVA for repeated measures and are presented as mean ± S.E.M. Each point represents six mice.
vehicle chronically treated mice could not be calculated because according to ALLFIT, a percent response greater than 25 was never attained.
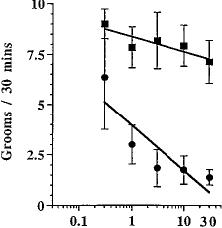
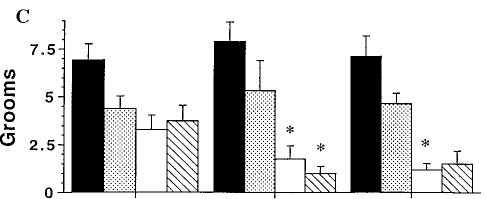
Upon challenge with SR141716A, the incidence of grooming behavior in mice treated chronically with O9-THC and those treated chronically with vehicle was markedly different (fig. 3C). Vehicle-vehicle treated mice exhibited 6.9 ± 0.8 (mean ± S.E.M.) grooming episodes during the 30-min observation period (table 1). A one-way ANOVA indicated that there was no significant effect of SR141716A in mice treated repeatedly with vehicle. However, O9-THC-treated mice showed a dose-dependent decrease in normal grooming behavior when challenged with the antagonist, SR141716A, generating an IC50 value of 0.80 mg/kg (0.32-2.0).
Unlike paw tremors and head shakes, the frequency of scratching was dose-responsive only for vehicle-treated mice (fig. 3D). Very little scratching was observed in mice chronically treated with O9-THC (10 mg/kg). A one-way ANOVA was done on each dose-response curve to determine if treatment differed from vehicle control. SR141716A dose-dependently increased scratching in mice treated chronically with vehicle with an ED50 value of 4.5 mg/kg (3.7-5.5). Table 1 summarizes the vehicle control mean ± S.E.M. values for the SR141716A dose-response curves for all four behaviors.
All of the data described above were generated with a chronic treatment regimen of 10 mg/kg of O9-THC. To determine whether the magnitude of the dependence syndrome was dependent upon the O9-THC treatment regimens, separate groups of mice were treated with either 3, 10 or 30 mg/kg of O9-THC or vehicle for 6.5 days and then challenged with an acute dose of either SR141716A (10 or 30 mg/kg) or vehicle on day 7 (fig. 4). The data were analyzed by using two-factor
10
60
C, c
t C)
M
40
20
N
Cannabinoid Dependence in Mice 1153
150
A
C
100
so
0
D
10
0 0
0.1 1 10 3 0 0.1 1 10 3 0
SR141716A (mg/kg, i.p.) SR141716A (mg/kg, i.p.)
observed in mice chronically treated with a dose of 3 mg/kg of O9-THC and challenged with vehicle (fig. 4). Planned comparisons showed no differences in the number of grooms in these mice when challenged with either vehicle or SR141716A. SR141716A markedly induced precipitated withdrawal in mice treated chronically with 10 mg/kg of O9-THC as indicated by a significant increase in the number of paw tremors and decrease in grooming behavior as compared with their respective chronic vehicle/SR141716A group. A significant difference also existed for all three behaviors between SR141716A- and vehicle-challenged mice that were treated chronically with a 10-mg/kg dose of O9THC.
Mice treated with 30 mg/kg of O9-THC exhibited physical withdrawal signs when challenged with 30 mg/kg of SR141716A. The frequency of paw tremors and head shakes increased although grooming behavior decreased upon administration of 30 mg/kg of SR141716A to the O9-THCtreated groups when compared with vehicle/SR141716A (30 mg/kg). However, when the challenge dose was lowered to 10 mg/kg of SR141716A, only grooming behavior exhibited any
* #
A
* #
* #
Fig. 3. SR141716A-precipitated withdrawal in mice. Mice received either Q9-THC (10 mg/kg) (•) or vehicle (1:1:18) (/) s.c. twice a day for 6 days and once on day 7. Four hours after the last injection, various doses of SR141716A or vehicle was administered i.p. The frequency of paw tremors (A), head shakes (B), grooming (C) and scratching (D) behavior was observed for 30 min immediately after the challenge drug or vehicle. Results are presented as mean ± S.E.M. Each point represents 6 to 17 mice.
TABLE 1
Vehicle effects after chronic vehicle or Q9-THC administration
|
Control Means ± S.E.M.a |
||||
|
Vehicle-vehicle treated |
O9-THC-vehicle treated |
|||
|
Paw tremors |
0.77 |
± 0.27 |
2.0 |
± 0.55 |
|
Head shakes |
1.0 |
± 0.32 |
0.76 |
± 0.27 |
|
Scratching |
4.6 |
± 1.5 |
3.4 |
± 0.77 |
|
Grooming |
6.9 |
± 0.84 |
3.3 |
± 0.76 |
|
Mice received either |
Q9-THC (10 mg/kg) or vehicle (1:1:18) s.c. twice a day for 6 |
|||
days and once on day 7. Four hours after the last injection, vehicle was administered i.p. The frequency of paw tremors, head shakes, grooming and scratching behavior was observed for 30 min immediately following the vehicle challenge.
a Control means ± S.E.M. represent 25 to 30 mice.
ANOVA. Bonferroni post hoc analyses were used when appropriate. A significant O9-THC regimen/SR141716A dose interaction resulted for both paw tremors (F = 7.854, P < .05) and head shakes (F = 2.446, P = .05). Although there was not a significant O9-THC dose/SR141716A dose interaction for grooming behavior (P = .3630), a main effect for O9-THC dose existed (F = 22.96, P < .05). SR141716A did not appear to precipitate physical withdrawal in mice treated chronically with 3 mg/kg of O9-THC, because neither dose of SR141716A elicited a significant increase in paw tremors in chronic O9THC/SR141716A mice compared to chronic vehicle/ SR141716A mice. O9-THC (3 mg/kg)/SR141716A did not differ significantly from its respective chronic vehicle/ SR141716A group for any of the behaviors reported. The number of paw tremors and head shakes observed in mice chronically treated with a dose of 3 mg/kg of O9-THC and challenged with SR141716A significantly differed from those
vehicle SR (10) SR (30)
Acute challenge (mg/kg)
Fig. 4. Influence of the Q9-THC treatment regimen on SR141716A-precipitated withdrawal. Mice were chronically treated with one of four s.c. dosing regimens: vehicle (/), 3 mg/kg of Q9-THC (~), 10 mg/kg of Q9-THC (O) or 30 mg/kg of Q9-THC (i) twice a day for 6 days. Four hr after the last chronic injection on day 7, mice received an acute i.p. administration of vehicle, 10 mg/kg of SR141716A [SR (10)] or 30 mg/kg of SR141716A [SR (30)]. Data were analyzed two-way ANOVA at P < .05 and Bonferroni post hoc (comparison with vehicle) analyses were used when appropriate. Results are presented as mean ± S.E.M. Each point represents 6 to 15 mice. *P < .05, compared to the respective chronic O9-THC/vehicle group. #P < .05, compared to respective chronic vehicle/SR141716A group.
la
100
50
*
a
0
B
SR (30)
*
* #
2
10 4 NNEIN"
vehicle
SR (30)

Vol. 285
difference. Compared with their respective chronic O9-THC/ vehicle group, mice treated with 30 mg/kg of O9-THC and challenged with 10 mg/kg of SR141716A had significantly more head shakes and less grooming behavior. When challenged with the higher dose of SR141716A (30 mg/kg), mice chronically treated with O9-THC (30 mg/kg) had significantly more paw tremors and head shakes compared to the chronic O9-THC/vehicle group as well as the chronic vehicle/ SR141716A group. In contrast, the apparent decrease in grooming behavior in these mice was not statistically significant from their respective control groups.
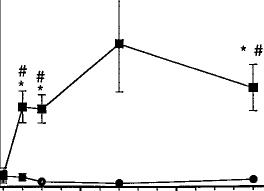
In summary, the existence of precipitated withdrawal in mice treated with O9-THC for approximately 1 wk was clearly dependent upon the doses of both O9-THC and SR141716A. To determine the time course for development of dependence, mice were treated with vehicle or O9-THC for either 1, 2, 3, 7 or 14 days and then challenged with SR141716A (fig. 5). A two-way factorial ANOVA revealed a drug effect (F = 72.782, P < .05), time effect (F = 4.757, P = .05), and interaction between number of days and challenge dose (F = 5.840, P = .05), with respect to paw tremors. The number of paw tremors increased as mice were exposed to a dose of 10 mg/kg of O9-THC for longer periods of time. Mice treated with O9-THC for either 2, 3, 7 or 14 days showed a significant increase in paw tremors compared with their respective vehicle group. The incidence of paw tremors was also greater in O9-THC-treated mice after 2, 3, 7 and 14 days compared with 1 day of O9-THC treatment. No main time effect (P = .3898) or interaction (P = .705) for head shakes
Days of A9-THC Administration
Fig. 5. Time course of dependence development in A9-THC-treated mice. Mice received either A9-THC (10 mg/kg) (f) or vehicle (1:1:18) (F) s.c. for either 1, 2, 3, 7 or 14 days. SR141716A (10 mg/kg) was administered i.p. 4 hr after the last A9-THC or vehicle injection on the test day. Behavior was observed for 30 min immediately after delivery of the challenge drug. Data were analyzed by ANOVA, P < .05 and Bonferroni post hoc analyses (comparison with vehicle). Results are presented as mean ± S.E.M. Each point represents 6 to 12 mice. *P < .05, compared to day 1. #P < .05, compared to the respective vehicle group.
was found in mice treated with O9-THC. There was however, a main drug effect (F = 22.738, P < .05) with respect to head shakes.
Discussion
Usually drug dependence can be established either by abruptly terminating chronic treatment and observing a spontaneous withdrawal syndrome or by precipitating a withdrawal syndrome in chronically treated animals with an appropriate antagonist. Abrupt withdrawal commonly occurs with drugs that do not have a long duration of action. It is reasonable to speculate that spontaneous withdrawal occurs if the biologically active levels of the drug dissipate before the endogenous system can fully recover from the dependent state. Not surprisingly, it is more difficult to detect spontaneous withdrawal with drugs, such as the cannabinoids, that have a long duration of action. Of course, the actions of both long- and short acting drugs can be abruptly terminated with an antagonist challenge.
Paw tremors appear to be a reliable indicator of cannabinoid dependence. In the present study, paw tremors were the most prominent and dose-responsive withdrawal sign observed. Others have reported involuntary paw tremors and twitching during SR141716A-precipitated withdrawal studies with rats (Tsou et al., 1995) as well as during abrupt withdrawal studies with rats and monkeys (Compton et al., 1990; Kaymakcalan, 1973). Head shakes were another common SR141716A-precipitated withdrawal sign observed in mice and appeared to be consistent with wet-dog shakes in rats that involve both head and body movements (Aceto et al., 1995, 1996; Tsou et al., 1995).
In abrupt and precipitated withdrawal studies of chronic cannabinoids, monkeys and rats exhibited an increase in motor activity (Beardsley et al., 1986; Kaymakcalan et al., 1977; Pertwee, 1991; Tsou et al., 1995) and excessive grooming (Kaymakcalan et al., 1977; Pertwee, 1991). Even though mice showed a remarkable decrease in grooming behavior and scratching during SR141716A-precipitated withdrawal, they still displayed a constantly changing disorganized sequence of movements very similar to that seen in rats during precipitated withdrawal (Tsou et al., 1995). The decrease in normal behavior in cannabinoid-dependent mice was most likely due to the fact that these mice were overwhelmingly preoccupied with paw tremors and head shakes. Paw tremors and other mouse behaviors, such as grooming, are mutually exclusive. Therefore, the mouse model of cannabinoid dependence resembles and confirms the rat model of cannabinoid precipitated withdrawal while adding credibility to the rat and monkey data from abrupt cannabinoid withdrawal studies.
Although mice and rats chronically exposed to O9-THC and then challenged with SR141716A elicited some common withdrawal signs, it is important to point out that scoring SR141716A-precipitated withdrawal was complicated by the fact that not all animals exhibit identical withdrawal behaviors. Some mice produced primarily paw tremors although head shakes dominated in others. A few mice exhibited little effect other than writhing. Even though strong similarities existed between rats and mice during SR141716A-precipitated withdrawal, differences among the withdrawal syndromes must also be considered. Observation of retropulsion
150
10 15
25 -
od
x
15 10
5
10
15
Cannabinoid Dependence in Mice 1155
and writhing was sporadic among individual mice which was in contrast to the more frequent observation of these behaviors in rats during SR141716A-precipitated withdrawal (Aceto et al., 1996; Tsou et al., 1995). Aceto et al. (1995, 1996) reported a marked appearance of eyelid ptosis and significant increase in facial rubbing in rats during SR141716Aprecipitated withdrawal. However, there was not a clear trend in the incidence of eyelid ptosis and facial rubbing in mice.
SR141716A alone had very little effect on naive mice with the exception of increased scratching. It significantly increased scratching in naive mice and dose-dependently increased scratching in vehicle-treated mice. Interestingly, scratching was suppressed in O9-THC-treated mice challenged with SR141716A. As mentioned above, perhaps the intensity of paw tremors and head shakes excluded the possibility of scratching. However, drug interaction between SR141716A and O9-THC cannot be ruled out. Because the O9-THC suppression of SR141716A induced scratching was not dose-dependent, a drug interaction appears unlikely. High doses of SR141716A elicited a few head shakes and paw tremors, although they were of low intensity and high variability. It is possible that SR141716A produces these effects either by disrupting the normal functioning of the endogenous cannabinoid system or by a direct pharmacological action of its own. The latter seems somewhat unlikely given that the pharmacological effects are identical with those observed during withdrawal.
There has been considerable interest in commonalities between opioids and cannabinoids, particularly with regards to tolerance and dependence. For example, cross-tolerance has been reported by Smith et al. (1994) between O9-THC and kappa opioid receptor agonists U-50,488H and CI-977. Evidence exists that suggests precipitation of withdrawal by naloxone or drug cessation in rats chronically exposed to O9-THC elicits behavioral signs suggestive of withdrawal (Hirschhorn and Rosecrans, 1974; Kaymakcalan et al., 1977; Verberne et al., 1980). In addition, O9-THC has been documented to suppress naloxone-induced precipitated withdrawal in morphine-dependent rats (Bhargava, 1976; Bhargava, 1978; Hine et al., 1975). Conversely, naloxone did not elicit any behavioral effects in monkeys chronically exposed to O9-THC (Beardsley et al., 1986) nor did SR141716A precipitate morphine withdrawal in morphine-dependent rats (Aceto et al., 1996). Cannabinoids and SR141716A clearly compete for receptor binding sites, whereas there has been no conclusive evidence to suggest that cannabinoids compete with naloxone binding. Therefore, SR141716A appears to be selective for cannabinoids. While it is clear that distinctive endogenous systems are involved in dependence to opioids and cannabinoids, the expression of withdrawal could result from activation of common neurochemical pathways.
For some classes of drugs, dependence intensity is a function of the degree of tolerance developed. However, O9-THC is capable of producing a considerable degree of tolerance, yet termination of chronic treatment is not accompanied by a severe withdrawal syndrome. Previous studies in our laboratory have shown pharmacological tolerance in mice after a dose of 10 mg/kg of O9-THC twice a day for 1 wk (Abood et al., 1993; Fan et al., 1994). It is particularly important to point out that this treatment regimen produces very few overt behavioral effects. The data from this study suggest that
using the same treatment regimen, physical dependence developed in addition to pharmacological tolerance. The data from this study as well as from other studies suggests that cannabinoid tolerance (Compton et al., 1990; Pertwee, 1991) and dependence could possibly develop as quickly as 2 days. However, parallel time course studies have not been conducted to establish the relationship between these two phenomena.
Before the discovery of SR141716A, dependence studies had to rely on abrupt cessation of chronic drug administration. This approach led to conflicting data that were difficult to interpret, especially in humans. Common withdrawal signs noted by several investigators were hyperirritability, tremors, sweating, dysphoria, anxiety, negativism, weight loss (decreased appetite) and insomnia (Cohen et al., 1976; Fraser, 1949; Georgotas and Zeidenberg, 1979; Greenberg et al., 1976; Jones and Benowitz, 1976; Jones et al., 1976, 1981; Mendelson et al., 1976; Soueif, 1967). A diminution of withdrawal signs was observed upon re-administration of O9-THC (Jones, 1983). However, a major confound with human studies was the anticipation of drug withdrawal.
The availability of SR141716A made it possible to carry out studies to either refute or reinforce the theory of physical dependence development to cannabinoids. Therefore, it was vital that precipitated withdrawal studies with cannabinoids be conducted. The data from the present investigation together with the previous dependence studies in rats (Aceto et al., 1995, 1996; Tsou et al., 1995) clearly demonstrated the development of cannabinoid dependence.
We were able to develop and characterize a mouse model for cannabinoid dependence. SR141716A induced a precipitated withdrawal syndrome that included involuntary paw tremors and head shakes, disorganized random movements and decrease in grooming and scratching behavior. Paw tremors, head shakes and grooming behavior were dependent on the dose of SR141716A. Moreover, this precipitated withdrawal syndrome could be precipitated in mice treated for only 2 days with O9-THC.
In summary, in mice as well as in humans (Jones, 1983), the intensity of the dependence depended on the length of treatment time and dose of O9-THC. It is important to keep in mind that the chronic O9-THC regimen used in the mouse model mimics heavy marijuana use. Because the frequency, quantity and duration of drug use dictate the intensity of dependence, it seems unlikely that infrequent marijuana use will result in dependence.
Acknowledgments
The authors thank Michael Dewey and Gray Patrick for their excellent technical assistance.
References
Abood ME, Sauss C, Fan F, Tilton CL and Martin BR (1993) Development of behavioral tolerance of Q9-THC without alteration of cannabinoid receptor binding or mRNA levels in the whole brain. Pharmacol Biochem Behav 46:575-579.
Aceto M, Scates S, Lowe J and Martin B (1995) Cannabinoid precipitated withdrawal by the selective cannabinoid receptor antagonist, SR 141716A. Eur J Pharmacol 282:R1–R2.
Aceto M, Scates S, Lowe J and Martin B (1996) Dependence on O9-tetrahydrocannabinol: Studies on precipitated and abrupt withdrawal. J Pharmacol Exp Ther 278:1290-1295.
Beardsley PM, Balster RL and Harris LS (1986) Dependence on tetrahydrocannabinol in rhesus monkeys. J Pharmacol Exp Ther 239:311-319.
Bhargava HN (1976) Effect of some cannabinoids on naloxone-precipitated abstinence in morphine-dependent mice. Psychopharm 49:267-270.
Bhargava HN (1978) Time course of the effects of naturally occurring cannabinoids on morphine abstinence syndrome. Pharmacol Biochem Behav 8:7-11.
1156 Cook et al.
Cohen S, Lessin P, Hahn PM and Tyrell ED (1976) A 94-day cannabis study, in Pharmacology of Marihuana (Braude MC and Szara S ed) pp 621-626, Raven Press, New York.
Compton D, Aceto M, Lowe J and Martin B (1996) In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): Inhibition of O9tetrahdrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther 277:586-594.
Compton DR, Dewey WL and Martin BR (1990) Cannabis dependence and tolerance production, in Addiction Potential of Abused Drugs and Drug Classes (Erickson KC, Javors MA and Morgan WW eds) pp 129-147, The Hayworth Press, Inc., Binghamton, NY.
Fan F, Compton DR, Ward S, Melvin L and Martin BR (1994) Development of cross-tolerance between Q9-THC, CP 55,940 and WIN 55,212. J Pharmacol Exp Ther 271:1383-1390.
Fan F, Tao Q, Abood M and Martin BR (1996) Cannabinoid receptor down-regulation without alteration of the inhibitory effect of CP 55,940 on adenylyl cyclase in the cerebellum of CP 55,940-tolerant mice. Brain Res 706:13-20.
Fraser JD (1949) Withdrawal symptoms in cannabis-indica addicts. Lancet 1:747748.
Georgotas A and Zeidenberg P (1979) Observations on the effects of four weeks of heavy marihuana smoking on group interaction and individual behavior. Compr Psych 20:427-432.
Greenberg I, Mendelson JH, Kuehnle JC, Mello N and Babor TF (1976) Psychiatric and behavioral observations of casual and heavy marijuana users in a controlled research setting. Ann NY Acad Sci 282:72-84.
Herkenham M, Groen BGS, Lynn AB, De Costa BR and Richfield EK (1991) Neuronal localization of cannabinoid receptors and second messengers in mutant mouse cerebellum. Brain Res 552:301-310.
Hine B, Friedman E, Torellio M and Gershon S (1975) Morphine-dependent rats: Blockade of precipitated abstinence by tetrahydrocannabinol. Science 187:443445.
Hirschhorn ID and Rosecrans JA (1974) Morphine and O9-tetrahydrocannabinol: Tolerance to the stimulus effects. Psychopharmacology 36:243-253.
Jones RT (1983) Cannabis tolerance and dependence, in Cannabis and Health Hazards (Fehr KO and Kalant H eds) pp 617-689, Addiction Research Foundation, Toronto, Canada.
Jones RT and Benowitz N (1976) The 30-day trip –Clinical studies of cannabis tolerance and dependence, in Pharmacology of Marihuana (Braude MC and Szara S eds) pp 627-642, Raven Press, New York.
Jones RT, Benowitz N and Bachman J (1976) Clinical studies of cannabis tolerance and dependence. Ann NY Acad Sci 282:221-239.
Jones RT, Benowitz NL and Herning RI (1981) Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol 21:143S–152S.
Kaymakcalan K, Ayhan IH and Tulunay FC (1977) Naloxone-induced or postwithdrawal abstinence signs in O9-tetrahydrocannabinol -tolerant rats. Psychopharmacology 55:243-249.
Kaymakcalan S (1973) Tolerance to and dependence on cannabis. Bull Narc 25:3947.
Kaymakcalan S (1979) Physiological and psychological dependence on the in rhesus monkeys, in Marihuana: Biological Effects. Advances in the Biosciences (Nahas GG and Paton WDM eds) pp. 142-149, Pergamon Press, Oxford.
Vol. 285
McMillan DE, Dewey WL and Harris LS (1971) Characteristics of tetrahydrocannabinol tolerance. Ann NY Acad Sci 191:83-99.
Mendelson J, Kuehnle J, Greenberg I and Mello N (1976) Operant acquisition of marihuana in man. JPET 198:42-53.
Pertwee R (1993) The evidence for the existence of cannabinoid receptors. Gen Pharmacol 24:811-824.
Pertwee R, Griffin G, Lainton J and Huffman J (1995) Pharmacological characterization of three novel cannabinoid receptor agonists in the mouse isolated vas deferens. Eur J Pharmacol 284:241-247.
Pertwee R, Stevenson L and Griffin G (1993) Cross-tolerance between delta-9tetrahydrocannabinol and the cannabimimetic agents, CP 55,940, WIN 55,212-2 and anandamide. Br J Pharmacol 110:1483-1490.
Pertwee RG (1991) Tolerance to and dependence on psychotropic cannabinoids, in The Biological Bases of Drug Tolerance and Dependence (Pratt JA ed) pp 231-263, Academic Press, New York.
Pertwee RG, Stevenson LA, Elrick DB, Mechoulam R and Corbett AD (1992) Inhibitory effects of certain enantiomeric cannabinoids in the mouse vas deferens and the myenteric plexus preparation of guinea-pig small intestine. Br J Pharmacol 105:980-984.
Rinaldi-Carmona M, Barth F, He´aulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Ne´liat G, Caput D, Ferrara P, Soubrie´ P, Brelie`re JC and Le Fur G (1994) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350:240-244.
Rinaldi-Carmona M, Pialot F, Congy C, Redon E, Barth F, Bachy A, Brelie`re JC, Soubrie´ P and Le Fur G (1996) Characterization and distribution of binding sites for [3H]-SR 141716A a selective brain (CB1) cannabinoid receptor antagonist in rodent brain. Life Sci 58:1239-1247.
Sjoden P (1973) Effects of long-term administration and withdrawal of tetrahydrocannabinols (O8-THC and Q9-THC) on open-field behavior in rats. Pharmacol Biochem Behav 1:243-249.
Smith PB, Welch SP, Martin BR (1994) interactions between O9-Tetrahydrocannabinol and kappa opioids in mice. J Pharmacol.
Soueif MI (1967) Hashish consumption in Egypt, with special reference to psychosocial aspects. Bull Narc 19:1-12.
Tallarida RJ and Murray RB (1987) Graded dose-response, in Manual of Pharmacologic Calculations, pp 26-31, Springer-Verlag, New York.
Taylor DA and Fennessy MR (1982) Time-course of the effects of chronic 09tetrahydrocannabinol on behavior, body temperature, brain amines and withdrawal-like behaviour in the rat. J Pharm Pharmacol 34:240-245.
Tsou K, Patrick S and Walker JM (1995) Physical withdrawal in rats tolerant to O9-tetrahydrocannabinol precipitated by a cannabinoid receptor antagonist. Eur J Pharmacol 280:R13–R15.
Verberne AJM, Taylor DA and Fennessy MR (1980) Withdrawal-like behaviour induced by inhibitors of biogenic amine reuptake in rats treated chronically with O9tetrahydrocannabinol. Psychopharmacol 68:261-267.
Send reprint requests to: Dr. Billy R. Martin, Department of Pharmacology and Toxicology, Medical College of Virginia, Virginia Commonwealth University, Box 980613, MCV Station Richmond, VA 23298-0613.
| Index |
| Index |
Last Updated (Monday, 20 December 2010 15:27)












