Abstinence symptoms following smoked marijuana in humans
Drug Abuse
© Springer-Verlag 1999
ORIGINAL INVESTIGATION
Margaret Haney · Amie S. Ward · Sandra D. Comer Richard W. Foltin · Marian W. Fischman
Abstinence symptoms following smoked marijuana in humans
Received: 2 June 1998 / Final version: 11 September 1998
Abstract Symptoms of withdrawal after oral A9tetrahydrocannabinol (THC) administration have been reported, yet little is known about the development of dependence on smoked marijuana in humans. In a 21day residential study, marijuana smokers (n = 12) worked on five psychomotor tasks during the day (0915–1700 hours), and in the evening engaged in recreational activities (1700–2330 hours); subjective-effects measures were completed 10 times/day. Food and beverages were available ad libitum from 0830 to 2330 hours. Marijuana cigarettes (0.0, 1.8, 3.1% THC) were smoked at 1000, 1400, 1800, and 2200 hours. Placebo marijuana was administered on days 1–4 . One of the active marijuana doses was administered on days 5–8, followed by 4 days of placebo marijuana (days 9–12). The other concentration of active marijuana cigarettes was administered on days 13–16, followed by 4 days of placebo marijuana (days 17–20); the order in which the high and low THC-concentration marijuana cigarettes were administered was counter-balanced between groups. Both active doses of marijuana increased ratings of “High,” and “Good Drug Effect,” and increased food intake, while decreasing verbal interaction compared to the placebo baseline (days 1–4). Abstinence from active marijuana increased ratings such as “Anxious,” “Irritable,” and “Stomach pain,” and significantly decreased food intake compared to baseline. This empirical demonstration of withdrawal from smoked marijuana may suggest that daily marijuana use may be maintained, at least in part, by the alleviation of abstinence symptoms.
M. Haney (®) · A.S. Ward · S.D. Comer · R.W. Foltin M.W. Fischman
Division on Substance Abuse, New York State Psychiatric Institute and Department of Psychiatry, College of Physicians and Surgeons of Columbia University, 722 West 168th Street, Unit 54, New York, NY 10032, USA e-mail: This e-mail address is being protected from spambots. You need JavaScript enabled to view it , Fax:+1-212-543-5991
Key words 3Marijuana · Dependence · Withdrawal
·
Human · Tolerance · Subjective effect
·
Performance · Residential laboratory
Introduction
The recent sharp rise in marijuana smoking, particularly in young men and women, has been accompanied by an increase in the number of people seeking treatment for marijuana abuse. Approximately 5% of high school seniors in the United States are reporting that they smoke marijuana daily (Johnston et al. 1995, 1997; Frank and Galea 1995). As with other “harder” drugs of abuse, a subset of marijuana users are also reporting uncontrolled marijuana use that is interfering with their daily lives. A significant proportion of individuals responding to advertisements targetting marijuana users report that they have been unable to stop smoking marijuana when they want to, and that they experience symptoms of withdrawal when they abstain from marijuana (Roffman et al. 1998; Stephens et al. 1993, 1994).
One possible reason that a subset of marijuana smokers are seeking treatment is that daily marijuana use can result in the development of physical dependence. In the United States, 7.4% of adult and 14.4% of adolescent marijuana users meet DSM-IV diagnostic criteria for marijuana dependence within the past year (Budney et al. 1997). Further, the development of cannabinoid dependence has been substantiated by laboratory studies. Precipitated withdrawal from A9tetrahydrocannabinol (THC), the major psychoactive component in marijuana, has been demonstrated in rats using the cannabinoid receptor antagonist, SR141716A (Rinaldi-Carmona et al. 1994): rats repeatedly exposed to THC showed dramatic symptoms of withdrawal, e.g., ptosis, wet-dog shakes, “anxiety” reactions, and disorganized patterns of motor
396
activity, when injected with SR141716A (Aceto et al. 1995; Tsou et al. 1995; de Fonseca et al. 1997). Abstinence from THC following a regimen of THC maintenance has also been shown to produce withdrawal signs. In rhesus monkeys, abstinence from intravenous (IV) THC resulted in aggression, hyperirritability, anorexia (Kaymakcalan 1973) and disruptions in operant behavior (Beardsley et al. 1986); the specificity of this latter effect was demonstrated by its reversal with IV THC administration.
In human laboratory studies, maintenance of high doses of oral THC (210 mg/day for 10–20 days), produced both tolerance and dependence (Jones et al. 1976, 1981). During THC administration, there was a progressive decline in THC’s cardiovascular and subjective effects. After abrupt cessation of THC administration, the majority of volunteers reported irritability, restlessness, decreased appetite, and sleep disturbances; re-administration of oral THC diminished most of these symptoms. Recent studies from this laboratory (Haney et al. 1999) demonstrate that abstinence symptoms also occur when human participants are exposed to lower daily doses of oral THC (80–120 mg/day) for a briefer period of time (3–4 days) than used in the Jones et al. studies (1976, 1981). During THC administration, there was a progressive decline in subjective effects ratings, while abstinence from THC increased ratings of anxiety, depression, and irritability, decreased the reported quantity and quality of sleep, and decreased food intake by 20–30% compared to baseline (Haney et al. 1999; Ward et al., in preparation).
Although the effects of oral THC cannot be expected to generalize fully to those of smoked marijuana, laboratory studies have demonstrated that abstinence following repeated marijuana smoking is also associated with a range of withdrawal symptoms. One research participant, who averaged nine marijuana cigarettes/day (1.8% THC) for 21 days, experienced symptoms of anxiety, dysphoria, anorexia and sweating upon cessation of marijuana use (Mendelson et al. 1984). Long-term inpatient studies of marijuana also provide clinical descriptions of irritability, hostility, restlessness, sleeplessness and loss of appetite during periods of marijuana abstinence (Georgotas and Zeidenberg Nowlan 1979; and Cohen 1977). Finally, a study using a laboratory model of human aggression (point-subtraction aggression paradigm; Cherek 1981), provides evidence that marijuana abstinence is associated with increased aggressive behavior. Frequent marijuana smokers abstinent from marijuana for up to 7 days showed greater levels of aggressive responding when provoked, compared to when they were still using marijuana (Kouri et al. 1998). These data suggest that the irritability manifested during marijuana abstinence may translate into aggressive behavior in the natural ecology.
The purpose of the present study was systematically to determine the effect of marijuana abstinence when
marijuana is smoked at levels comparable to those reportedly used in natural settings. Specifically, the effects of marijuana and marijuana abstinence on a range of behaviors, mood, food intake, sleep, social behavior, and performance on psychomotor and memory tasks, were measured.
Materials and methods
Participants
Twelve male (seven African-American, three Non-Hispanic Caucasian, two Hispanic) healthy research volunteers ranging in age from 21 to 44 years (mean: 28), participated in a 21-day experiment. Prior to study participation, volunteers provided a detailed drug and medical history, received complete medical and psychiatric evaluations, and signed consent forms detailing all aspects of the research. Participants reported smoking marijuana 5.8 ± 0.4 days/week (mean ± SD), averaging 6.7 ± 1.6 marijuana cigarettes per day. Most participants also reported drinking alcohol weekly (mean: 2 day/week, two drinks/occasion). Eight reported smoking tobacco cigarettes, and continued to do so during the experiment. Other drug use was infrequent, although one subject reported occasional cocaine use. Participants did not diet, were within accepted weight ranges for their heights [69.8 ± 1.7kg (Metropolitan Life Insurance Company 1983)], and had no self-reported eating abnormalities.
Participants were instructed that they were participating in a study of the behavioral effects of marijuana. They were told that the strength of the marijuana cigarettes might change at any time. Prior to discharge, participants were fully informed about the experimental and drug conditions. All procedures were approved by the New York State Psychiatric Institute’s Institutional Review Board.
Laboratory
Participants, in three groups of four, lived in a residential laboratory designed for the continuous observation of human behavior over extended periods of time. The residential laboratory consists of 11 rooms in the New York State Psychiatric Institute: four private participant rooms, a common recreational area, two singleoccupancy bathrooms, two single-occupancy shower rooms, and two vestibules used for exchanging supplies (see Haney et al. 1998, for a more detailed laboratory description).
Output from a video and audio monitoring system terminated in an adjacent control room. Participants were observed continuously except while in the bathroom or in private dressing areas. No video or audio recordings were made. Communication between participants and experimenters was accomplished using a networked computer system, linking each participant’s computer with the computer in the main control room and allowing for a continuous on-line interaction between participants and experimenters, but not between participants.
Procedure
Prior to residence, participants received two training sessions (3– 4 h/session) on the computerized tasks and on a separate day, smoked a marijuana cigarette (3.1% THC). Participants moved into the laboratory on the day before the study, during which they received additional training on tasks and experimental procedures. The first experimental day began at 0830 hours the following morning. The St Mary’s Hospital Sleep Questionnaire, rating the
previous night’s sleep, was completed each morning. Participants first completed a 50-item visual analog scale (VAS), which consisted of a 100-mm line anchored with “not at all” at the left end and “extremely” at the right end, labeled with “I feel...” “High,” “Stimulated,” “Anxious,” “Sedated,” “Depressed,” “Hungry,” “Friendly,” “Miserable,” “On edge,” “Alert,” “Tired,” “Talkative,” “Self-confident,” “Social,” “Irritable,” “Confused,” “A good drug effect,” “A bad drug effect,” “Dizzy,” “Sleepy,” “Like yawning,” “Energetic,” “Jittery,” “Content,” “Unmotivated,” “Restless,” “Nauseated,” “Like vomiting,” “Suicidal,” “Forgetful,” “Mellow,” “Clumsy,” “Numbness or tingling in my extremities,” “Withdrawn,” “I have...”. “An upset Stomach,” “Muscle pain,” “A runny nose,” “A headache,” “Flu-like symptoms,” “Chills,” “Goose flesh,” “Stomach pain,” “I’m having difficulty concentrating,” “I am sweating,” “I’m having difficulty sleeping,” “I am dreaming more,” “My heart is pounding or beating faster that usual,” “Noises or sounds seem louder than usual,” “My vision is blurred,” and “My limbs feel heavier than usual.’’ Participants were then weighed and given time to eat breakfast. Three work periods occurred each day: The first work period (0915–0945 hours), consisted of one task battery, composed of five computer tasks and the VAS. Marijuana was first smoked at 1000 hours each day. Participants then began their second work period (1015–1315 hours), composed of four task batteries; each task battery consisted of the same five tasks and the VAS. The Drug-Effects Questionnaire was completed on the computer 90min after each administration of marijuana. The second administration of marijuana occurred at 1400 hours. The final work period (1415–1615 hours) consisted of another three task batteries. Beginning at 1715 hours, participants had access to activities available in the recreation area. Marijuana was smoked for the third time each day at 1800 hours. Two video-taped films were shown, one beginning at 1815 hours and the other at 2115 hours. The final marijuana administration occurred at 2200 hours. At 2330 hours, the recreation area was no longer available. A final VAS and a marijuana withdrawal checklist (modified from a cocaine withdrawal checklist; Brower et al. 1988), in which participants answered if they did or did not experience a range of symptoms, were completed at 2330 hours. Lights were turned off no later than 2400 hours.
Food
Every morning at 0830 hours each participant received a box of food containing a variety of meal items, snacks and beverages which could be consumed at any time within the day. Frozen meal items were also continuously available by request. To facilitate choice of frozen meals, participants were provided with a book containing package pictures of each item. Additional units of any item were freely available upon request. Participants were instructed to scan custom-designed bar codes whenever they ate or drank, specifying substance and portion. At 2330 hours, participants returned their food box to a staff member. Food items were not available between 2330 hours and 0830 hours the following morning, although water was available at all times.
Task battery
Each task battery consisted of a 3-min digit-symbol substitution task (DSST), a 3-min repeated acquisition task, a 10-min divided attention task (DAT), a 10-min rapid information task (RIT), an immediate and delayed digit-recall task, and a VAS. The battery measures various aspects of learning, memory, vigilance and psychomotor ability (see Foltin et al. 1996, for description of the tasks). Participants were instructed to complete each task as quickly and as accurately as possible.
397
Social behavior
A computerized observation program was used to record behavior categorically every 2.5 min during each evening recreation period. Behaviors were divided into two categories: private and social. Private behaviors occurred in each participant’s private room or in the bathroom/shower room. Social behaviors occurred in the recreation area. Social behavior was further categorized as being either verbal or non-verbal.
Marijuana administration
Participants received two marijuana cigarettes (0, 1.8, 3.1% THC weight/weight, provided by the National Institute on Drug Abuse) prior to each scheduled smoking occasion. Marijuana was administered using a cued-smoking procedure, which has been shown to produce reliable increases in heart-rate and plasma levels of A9THC (Foltin et al. 1987). Colored lights (mounted on the ceiling of the social area) signalled “light the cigarette” (30 s), “get ready” (5 s), “inhale” (5 s), “hold smoke in lungs” (10 s) and “exhale”. Participants smoked five puffs (three puffs on one cigarette, two on the other) in this manner, with a 40-s interval between each puff. Participants were instructed that they could signal that they wanted to stop smoking by raising their left hand, yet no participant did. Since participants can discriminate THC content by the color of the plant material (Chait and Pierri 1989), cigarettes were tightly rolled at both ends and were smoked through a hollow plastic cigarette holder so the marijuana was not visible. Cigarettes were stored frozen in an airtight container and humidified at room temperature for 24 h prior to use.
Tobacco cigarette smoking
The social and private areas are equipped with pressure-activated sensors through which all tobacco cigarettes are smoked. The sensors are connected to color-coded plastic cigarette holders with PVC tubing in order to allow recording of each cigarette puff by each participant.
Design
Marijuana cigarettes were administered four times/day at 1000, 1400, 1800 and 2200 hours. During the first 4 inpatient days, participants received placebo marijuana. On days 5–8, one of the active marijuana concentrations was administered, followed by 4 days of placebo marijuana (days 9–12). The other active marijuana concentration was administered on days 13–16, followed by 4 days of placebo (days 17–20); the order in which the 1.8% and 3.1% THC concentration marijuana cigarettes were administered was counterbalanced between studies. Participants moved out on day 21.
Data analysis
Repeated measures analyses of variance (ANOVA) with planned comparisons were used to address two issues: The first was to determine the effect of repeated marijuana exposure and abstinence from marijuana on subjective effects (peak daily ratings), drug effects (peak daily ratings), task performance, social behavior, food intake [total energy intake, g-intake of carbohydrate, fats and protein, percent of energy intake derived from each macronutrient estimated as kcal from g-intake using Atwater factors (McLaren 1976)], and body weight. The first 4 days of placebo baseline (days 1–4) were compared to 4 days of each later condition. Thus, there were two within-group factors [Condition (baseline, 1.8%, 3.1%, post-1.8%,
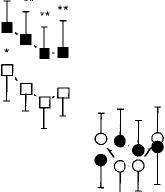
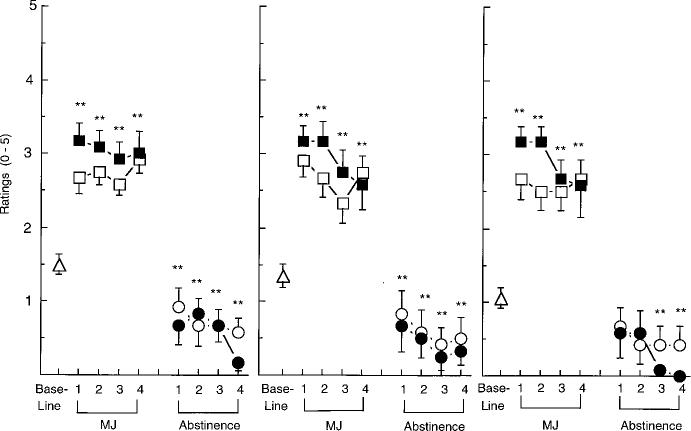
Fig. 1 Selected peak
subjective-effects ratings under each active marijuana condition and during the
abstinence period that 100
followed each active marijuana condition as a function of day of condition. The single open triangle on each graph
represents the mean peak rating observed during the 4 days of the initial baseline. Number signs indicate a significant difference between days 1 and 4 within each condition (#P < 0.01; ##P < 0.005), and asterisks indicate a significant difference
between that day and baseline 25
(*P < 0.01; **P < 0.005). Error bars represent ±
standard error of the mean (SEM). A Baseline, 0 1.8% THC, ∎ 3.1% THC, O Abstin 1.8% THC, • Abstin 3.1% THC
398
post-3.1%) and Day of Condition (1, 2, 3, 4)]. Sixteen planned comparisons were completed for each measure: baseline was compared to each THC concentration (1.8%, 3.1%) and baseline was compared to each abstinence condition (post-1.8%, post-3.1%) for the 4 days of each condition. Analysis of certain food data included an additional within-group condition: time of day: 0–1259 (including breakfast); 1300–1659 (including lunch) and 1700–2330 hours (including dinner). For these data, 16 planned comparisons between baseline and each dose condition were made at the three times of day; the days of each condition were collapsed. A second objective was to determine if tolerance developed to the effects of repeated marijuana administration. For this comparison, two planned comparisons were made: peak ratings occuring on the first and last day of the 1.8% THC and the 3.1% THC condition were compared. Given the large number of planned comparisons overall, only those with P values less than 0.01 were considered statistically significant, in an effort to control for type I error. Hunyh-Feldt corrections were used, when appropriate.
Results
Subjective-effects ratings
Each figure and table portrays the peak baseline measure averaged across the 4-day initial placebo period, followed by the peak data for each day of active marijuana administration, and each day of marijuana abstinence. Fig. 1 illustrates that both concentrations of THC significantly increased ratings of “Good Drug Effect,” “High” and “Stimulated” compared to baseline. Tolerance developed to the effects of the 3.1% THC cigarettes, evidenced by a significant diminution of peak ratings of “Good Drug Effect” and “High” on day 4 of its administration compared to day 1 of administration. During abstinence from either marijuana con
dition, participants rated the placebo cigarettes as giving them less of a “Good Drug Effect,” and making them less “Stimulated” compared to the placebo cigarettes at baseline.
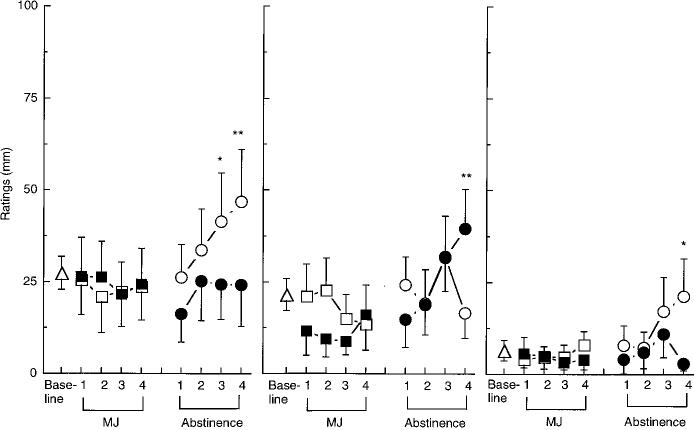
As shown in Fig. 2, ratings of “Anxiety” were significantly increased on the third and fourth day of abstinence from the 1.8% THC cigarettes compared to the baseline condition. Ratings of “Stomach Pain” and “Irritability” were increased on day 4 of abstinence from the 1.8% and 3.1% THC cigarettes, respectively. Additional significant effects of drug condition on mood are portrayed in Fig. 3: Both active marijuana conditions increased ratings of “Mellow” on each day of administration, while abstinence from either active concentration decreased ratings of “Mellow” on days 3 and 4 of abstinence. Similarly, ratings of “Content” and “Friendly” were significantly decreased during abstinence from either marijuana condition.
Additional significant effects are reported in Table 1. Both active marijuana conditions decreased ratings of “On Edge” (Table 1) and increased ratings of “Clumsy” [1.8%, day 1: F1,12=11.81; day 2: F1,12=13.01; day 4; 3.1%, day 2: F1,12=14.49, P<0.01 (data not shown)] compared to baseline. The 1.8% THC cigarettes increased ratings of “Talkative,” while the 3.1% THC cigarettes increased ratings of “Noises seem Louder than Usual,” “Heart Pounding more than Usual (Table 1),” and “Blurred Vision” [day 3: F1,12=10.21, P<0.01 (data not shown)]; marijuana had significantly less of an effect on heart pounding by day 4 of administration compared to day 1 (Table 1). During abstinence from the 3.1% THC condition, ratings of “Talkative” were decreased, while ratings of “Social” and “Energetic” were significantly decreased
SUBJECTIVE EFFECTS
"Good Drug Effect"
"High"
"Stimulated"
75
R
50
#n
a b b
I t`%. ,, 1 1 1 I 7' 7-16'o I I I I I 1 I I I
1 2 3 4 Base- 1 2 3 4 1 2 3 4 Base- 1 2 3 4 1 2 3 4
I l Line i I I I Line i I I I
Abstinence MJ Abstinence MJ Abstinence
0
Base- 1 2 3 4
Line I I
MJ
DAYS
399
Fig. 2 Selected peak subjective-effects ratings. See Fig. 1 legend for details
Fig. 3 Selected peak subjective-effects ratings. See Fig. 1 legend for details
"Anxious"
SUBJECTIVE EFFECTS
"Irritable"
"Stomach Pain"
"Mellow"
100 75 50 25
SUBJECTIVE EFFECTS
"Content"
"Friendly"
0 ''''' I i i i ''' i i''' i i i t i''' i i i I
Base- 1 2 3 4 1 2 3 4 Base- 1 2 3 4 1 2 3 4 Base- 1 2 3 4 1 2 3 4
Line I Line I Line I I
MJ Abstinence MJ Abstinence MJ Abstinence DAYS
during abstinence from either marijuana condition from marijuana, participants were less willing to smoke (Table 1). the placebo marijuana cigarettes again compared to
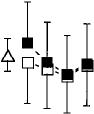
baseline (Fig. 4). Ratings of marijuana strength, and
liking (Fig. 4) were also significantly less during mar
Drug-effects questionnaire ijuana abstinence than at baseline.
Figure 4, portraying ratings on the Drug-Effects
Questionnaire, shows that marijuana significantly Marijuana withdrawal checklist increased ratings of marijuana strength, willingness to
smoke that concentration cigarette again, and liking There were no significant changes in response as a func
for the concentration of marijuana. During abstinence tion of drug condition.
400
Table 1 Means (± SEM) of peak subjective-effects ratings following 4 days of marijuana administration or abstinence from 4 days of marijuana administration
|
Days |
1 |
2 |
3 |
4 |
|||||
|
‘‘On Edge’’ 1.8% |
17.5 |
(8.3) |
6.2 |
(2.9)** |
13.9 |
(6.9) |
Placebo = 25.8 (4.5) |
||
|
11.2 (4.9) |
|||||||||
|
3.1% |
23.1 |
(10.1) |
6.2 |
(4.7)** |
2.7 |
(1.7)** |
4.6 (3.6)** |
||
|
Abstinence 1.8% |
16.4 |
(5.6) |
19.5 |
(6.7) |
24.3 |
(9.1) |
23.4 (11.1) |
||
|
Abstinence 3.1% |
11.2 |
(7.1) |
17.0 |
(8.7) |
19.9 |
(9.4) |
26.7 (10.5) |
||
|
‘‘Talkative’’ 1.8% |
68.6 |
(9.0)* |
63.9 |
(8.6) |
59.2 |
(7.4) |
Placebo = 57.4 (3.5) |
||
|
59.6 |
(7.8) |
||||||||
|
3.1% |
55.5 |
(8.9) |
51.0 |
(9.3) |
54.7 |
(8.6) |
50.9 |
(9.2) |
|
|
Abstinence 1.8% |
49.7 |
(8.5) |
47.2 |
(9.2) |
53.2 |
(8.6) |
57.7 |
(8.2) |
|
|
Abstinence 3.1% |
44.2 |
(9.2)** |
49.2 |
(8.4) |
50.9 |
(7.5) |
49.3 |
(8.0) |
|
|
‘‘Noises seem Louder’’ |
Placebo = 8.5 |
(2.1) |
|||||||
|
1.8% |
14.4 |
(6.6) |
8.8 |
(4.8) |
11.7 |
(5.7) |
11.9 |
(6.5) |
|
|
3.1% |
27.1 |
(9.3)** |
19.1 |
(7.3)* |
21.2 |
(9.4)* |
18.1 |
(9.1) |
|
|
Abstinence 1.8% |
5.3 |
(4.1) |
3.7 |
(3.4) |
2.8 |
(2.60) |
4.6 |
(3.3) |
|
|
Abstinence 3.1% |
7.2 |
(4.5) |
14.6 |
(8.8) |
7.1 |
(4.7) |
7.2 |
(4.6) |
|
|
‘‘Heart Pounding’’ |
Placebo = 3.6 |
(1.3) |
|||||||
|
1.8% |
11.1 |
(5.3) |
3.5 |
(1.8) |
4.2 |
(2.3) |
7.3 (4.2) |
||
|
3.1% |
20.2 |
(8.5)** |
8.0 |
(4.3) |
11.9 |
(5.8) |
2.1 (1.7)## |
||
|
Abstinence 1.8% |
2.9 |
(2.2) |
1.4 |
(0.9) |
0.4 |
(0.4) |
0.3 (0.3) |
||
|
Abstinence 3.1% |
5.9 |
(3.6) |
2.5 |
(2.0) |
2.1 |
(1.9) |
1.5 (1.0) |
||
|
‘‘Energetic’’ 1.8% |
61.3 |
(10.1) |
52.2 |
(9.8) |
48.6 |
(8.4) |
Placebo = 55.0 (3.9) |
||
|
45.6 (9.1) |
|||||||||
|
3.1% |
58.8 |
(10.8) |
54.4 |
(10.9) |
55.7 |
(8.9) |
54.1 (10.4) |
||
|
Abstinence 1.8% |
41.3 |
(9.6)** |
49.6 |
(10.2) |
47.6 |
(10.1) |
59.2 (10.0) |
||
|
Abstinence 3.1% |
47.5 |
(9.3) |
47.2 |
(6.9) |
47.1 |
(8.9) |
42.2 (8.8)** |
||
|
‘‘Social’’ 1.8% |
68.8 |
(7.5) |
58.3 |
(9.9) |
62.6 |
(9.2) |
Placebo = 61.0 (3.8) |
||
|
57.4 (9.1) |
|||||||||
|
3.1% |
55.3 |
(10.5) |
58.2 |
(9.1) |
50.0 |
(9.7) |
51.3 (9.8) |
||
|
Abstinence 1.8% |
48.8 |
(9.8) |
48.4 |
(10.3)* |
48.2 |
(9.5)* |
50.4 (9.6) |
||
|
Abstinence 3.1% |
44.0 |
(11.1)** |
48.6 |
(10.0)* |
53.7 |
(9.2) |
50.2 (8.9) |
||
Asterisks indicate a significant difference between the day of each condition and baseline (*P<0.01, ** P<0.005). Number signs indicate a significant difference between days 1 and 4 within each condition (# P<0.01, ## P<0.005)
St. Mary’s Hospital Sleep Questionnaire
There were no significant changes in response as a function of drug condition.
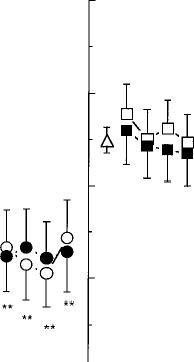
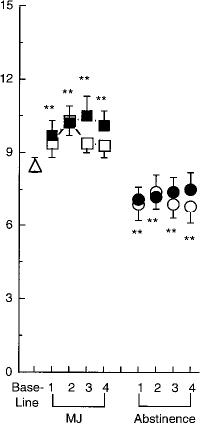
Food intake
As shown in Fig. 5, daily caloric intake was increased by both the 1.8% and 3.1% THC cigarettes on each day of administration; food intake was significantly increased in the evening for 1.8% THC cigarettes [F1,8=15.17, P<0.0002 (data not shown)]. Fig. 5 also shows that the mechanism by which marijuana influenced daily caloric intake was by increasing the number of eating occasions, defined as beginning with the first report of an item to be consumed and ending when there was a pause of greater than 10 min between food reports (Foltin et al. 1996). Abstinence from either active marijuana condition decreased total daily caloric intake by decreasing the number of eating occasions throughout the day. During abstinence from the 1.8% THC cigarettes, caloric intake in the evening was significantly decreased (data not shown), and partici
pants derived a significantly larger percentage of their total caloric intake from proteins (13.1% versus 11.8%) compared to baseline [day 1: F1,12=7.55, P < 0.01 (data not shown)].
Body weight significantly increased during active marijuana conditions compared to baseline: weights ranged from 71.2 to 71.7 kg on days 3 and 4 of each active marijuana condition, which were significantly higher than the average baseline weight of 69.6 ± 0.8 kg. Body weight did not significantly differ from baseline during either abstinence condition. By the end of the study, participants averaged a 0.9 kg weight gain compared to baseline levels.
|
Social behavior Both the 1.8% (day 3: F1,132=16.11; day 4: |
||
|
F1,132 = 14.64, P < 0.006) and the 3.1% THC (day 2: |
||
|
F1,132 = |
15.19; |
day 3: F1,132 = 30.59, day 4: |
|
F1,132 = |
36.20, |
P < 0.006) cigarettes significantly |
decreased the amount of time participants spent talking while they were in the social area. Under baseline conditions, participants spent an average of 30.9 ± 2.6%
401
Fig. 4 Selected peak ratings on the Drug-Effects Questionnaire. See Fig. 1 legend for details
Fig. 5 Mean daily caloric intake and mean number of eatings occasions. See Fig. 1 legend for details
"Strength"
Eating Occasions
U Y
of their time in the social area talking, while under active marijuana conditions, the percentage of time spent talking ranged from 4.2 to 13.6%, depending on the day of the condition. Abstinence from THC did not have a significant effect on time spent talking compared to the baseline condition.
Performance effects
Performance on the DSST was impaired following administration of the 3.1% THC cigarettes: partici
pants entered fewer patterns correctly compared to baseline [day 1: F1,132 = 12.28; day 2: F1,132 = 12.54; day 4: F1,132 = 16.92, P < 0.01 (data not shown)]. Performance on the DAT was improved on the first day the 1.8% THC cigarettes were administered, with participants achieving a higher maximum speed of entering the patterns than during baseline (day 1: F1,132 = 7.64, P < 0.006). Participants were less accurate in tracking the DAT’s moving target when abstinent from either the 1.8% (day 4: F1,132 = 23.58, P < 0.002) or the 3.1% (day 2: F1,132 = 16.70; day 4:
402
F1,132 = 16.50, P < 0.007 (data not shown)] THC cigarettes, compared to baseline.
Tobacco cigarette smoking
Data on only six of the eight cigarette smokers were available, due to equipment malfunction. The number of puffs cigarette smokers took on tobacco cigarettes did not vary as a function of drug condition: an average of 97 ± 39 cigarette puffs were taken over the course of the 4-day baseline condition. On days of 1.8% THC cigarette administration, 102±36 puffs were taken, while in the 3.1% THC condition, 111±33 puffs were taken. An average of 111±34 puffs were taken during abstinence from 1.8% THC cigarettes, and an average of 87 ± 28 puffs during abstinence from 3.1% THC cigarettes.
Discussion
The present study provides empirical evidence that abstinence from marijuana is associated with increases in anxiety, irritability and stomach pain, and decreases in the amount and frequency of food intake. These symptoms are similar to the pattern of abstinence effects following oral THC administration (Jones et al. 1976, 1981; Haney et al. 1999), and are similar to the effects seen in laboratory participants smoking marijuana repeatedly for at least 3 weeks (Nowlan and Cohen 1977; Georgotas and Zeidenberg 1979; Mendelson et al. 1984). The present findings are also consistent with interview data obtained in daily marijuana smokers, who report feeling ‘‘nervous, tense, and restless’’ when abstinent from marijuana (Wiesbeck et al. 1996).
Although there were no significant self-reports of sleep disturbance during marijuana abstinence, we have recently completed a study with portable sleep monitors (Respironics, Atlanta, Go., USA), showing that abstinence following exposure to four to five marijuana cigarettes/day (1.8–3.1% THC) for even a shorter period of time (3 days) was associated with a substantially increased latency to fall asleep and latency to rapid eye movement (Ward et al., in preparation). These data, using a more sensitive measure of sleep than selfreport, suggest that sleep disruption may be an additional consequence of marijuana abstinence.
For most measures, the mood changes during marijuana abstinence became statistically significant on the third or fourth day since the last marijuana cigarette, while changes in food intake occurred on day 1 and were statistically significant through day 4 of abstinence. How long these symptoms persist is unclear, since the abstinence condition was not maintained longer than 4 days. Although earlier data with oral
THC and smoked marijuana indicated that abstinence symptoms abated within 4 days (Jones et al. 1976; Mendelson et al. 1984), the fact that most symptoms observed in this study were peaking on day 4 suggests they would persist for a longer period of time.
Further, aggressive behavior is heightened for at least 7 consecutive days of marijuana abstinence (Kouri et al. 1998), supporting the idea that some symptoms, such as irritability, persist longer than 4 days of abstinence from marijuana.
Given the negative mood and behavioral symptoms associated with marijuana abstinence following daily marijuana exposure, it appears likely that the onset of abstinence symptoms may partly maintain chronic marijuana use, i.e. people continue to smoke marijuana each day because abruptly stopping is associated with negative mood. A similar pattern has been reported with nicotine: data from cigarette smokers indicate that nicotine deprivation leads to smoking in order to reverse the effects of withdrawal (USDHHS 1988; Heishman 1994). It may be that individuals who have a history of using drugs such as marijuana to modulate mood are particularly likely to increase their drug use as a consequence of even subtle changes in mood.
It is important to note that the subtle symptoms of marijuana abstinence observed in the present study could be dissociated from the development of tolerance. The literature is replete with studies, including our own, demonstrating that repeated exposure to high levels of marijuana results in tolerance to certain of its effects (Babor et al. 1975; Nowlan and Cohen 1977; Haney et al. 1997b). Similarly, in the present study, repeated exposure to high THC concentration marijuana cigarettes resulted in a significant decrease in three subjective-effects ratings (‘‘High,’’ ‘‘Good Drug Effect,’’ ‘‘Heart Pounding’’). However tolerance to the low THC concentration cigarettes did not develop under these conditions, although abstinence from the low THC concentration cigarettes also resulted in symptoms of withdrawal, such as anxiety and stomach pain. These data demonstrate that when behavior is carefully and continuously monitored, evidence of marijuana withdrawal can be detected even at levels of marijuana exposure that do not produce tolerance.
It is possible that this population of near-daily marijuana smokers were tolerant to many of marijuana’s behavioral effects even before starting the experimental protocol. Supporting this idea is the demonstration that marijuana had relatively minor effects on task performance (and in fact improved performance on the DAT). Studies directly comparing different populations of marijuana smokers have shown that heavy marijuana users show fewer performance decrements after smoking marijuana compared to light users (Meyer et al. 1971; Rickles et al. 1973; Cohen and Rickles 1974), presumably because the heavy users are tolerant to the impairing effects of marijuana. It is also probable that the present group of marijuana users were already
dependent on marijuana when they started the study. Thus, the period considered ‘‘baseline’’ was actually a period of marijuana abstinence. It was difficult to avoid this problem, since we did not want to expose lighter marijuana users to the dose regimen used in the present study, and increasing the length of the baseline placebo period would have made the duration of the residential study prohibitively long to most of our subject pool. Our rationale was that this design was in fact conservative, since it would minimize rather than amplify the likelihood that we would see a significant difference between the initial ‘‘baseline’’ period and the abstinent condition. In fact, initial baseline ratings of ‘‘Anxiety’’ and ‘‘Irritability’’ were 3–6 times higher in the present study compared to previous studies in this laboratory in individuals who did not smoke marijuana (Haney et al. 1997a, unpublished data) or in marijuana users who did not undergo a period of abstinence (Haney et al. 1997b, unpublished data), suggesting that there may be some indication of abstinence symptoms during the ‘‘baseline’’ period. We postulate that participants were experiencing some abstinence symptoms in the initial baseline period, but the symptoms became more robust when the amount and frequency of marijuana smoking was experimentally controlled between individuals, thereby decreasing variability.
To conclude, a distinct withdrawal syndrome can be observed following a relatively brief period of controlled marijuana administration in regular users of marijuana. Although adolescents are reporting a lower perceived risk of regular marijuana use compared to older adults (Johnston et al. 1997), the present data suggest that dependence may in fact be an important consequence to repeated, daily exposure to cannabi-noids. While not as dramatic as the withdrawal syndrome observed in individuals dependent on opioids, alcohol or barbiturates, this pattern of symptoms may still be significant to the individual marijuana user. This empirical demonstration of withdrawal from smoked marijuana, in combination with the epidemiological evidence for increased marijuana use, suggests that effective treatments targeting marijuana abuse may be needed.
Acknowledgements The assistance of Shannon Lewis, Joaquin Morales, Jessica Keitlen, Paul Toth, and Drs. Adam Bisaga, Maria Sullivan, Eric Collins, and Eric Rubin are gratefully acknowledged. This research was supported by US National Institute on Drug
Abuse grant DA03476–11 (M.W.F.) and the Aarn Diamond Fellowship Foundation (M.H.).
References
Aceto MD, Scates SM, Lowe JA, Martin BR (1995) Cannabinoid precipitated withdrawal by the selective cannabinoid receptor antagonist, SR 141716A. Eur J Pharmacol 282:R1–R2
Babor TF, Mendelson JH, Greenberg I, Kuehnle JC (1975) Marijuana consumption and tolerance to physiological and subjective effects. Arch Gen Psychiatry 32:1548–1552
403
Beardsley PM, Balster RL, Harris LS (1986) Dependence on tetrahydrocannabinol in rhesus monkeys. J Pharmacol Exp Ther 239:311–319
Brower KJ, Maddahian E, Low FC, Beresford TP (1988) A comparison of self-reported symptoms and DSM-III-R criteria for cocaine withdrawal. Am J Drug Alcohol Abuse 14:347–356
Budney AJ, Kandel DB, Cherek DR, Martin BR, Stephens RS, Roffman R (1997) College on Problems of Drug Dependence Meeting, Puerto Rico (June, 1996): marijuana use and dependence. Drug Alcohol Depend 45:1–24
Chait LD, Pierri J (1989) Some physical characteristics of NIDA marijuana cigarettes. Addict Behav 14:61–67
Cherek DR (1981) Effects of smoking different doses of nicotine on human aggressive behavior. Psychopharmacology 75:339–345
Cohen MJ, Rickles WH Jr (1974) Performance on a verbal learning task by subjects of heavy past marijuana usage. Psychopharmacologia 37:323–330
de Fonseca FR, Carrera MRA, Navarro M, Koob GF, Weiss F (1997) Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science 276:2050–2053
Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD (1987) Marijuana and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav 28:459–464
Foltin RW, Haney M, Comer SD, Fischman MW (1996) Effect of fluoxetine on food intake of humans living in a residential laboratory. Appetite 27:165–181
Frank B, Galea J (1995) Epidemiologic trends in drug abuse v. II. NIDA US Dept Health and Human Services, Washington, D.C.
Georgotas A, Zeidenberg P (1979) Observations on the effects of four weeks of heavy marijuana smoking on group interaction and individual behavior. Comp Psychiatry 20:427–432
Haney M, Comer SD, Foltin RW, Fischman MW (1997a) Behavioral contingencies modulate alprazolam self-administration by humans. Behav Pharmacol 8:82–90
Haney M, Comer SD, Ward AS, Foltin RW, Fischman MW (1997b) Factors influencing marijuana self-administration by humans. Behav Pharmacol 8:101–112
Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW (1999) Abstinence symptoms following oral THC administration to humans. Psychopharmacology 141:385–394
Heishman SJ, Taylor RC, Henningfield JE (1994) Nicotine and smoking: a review of effects on human performance. Exp Clin Psychopharmacol 2:345–395
Johnston LD, O’Malley PM, Bachman JG (1995) National survey results on drug use from the monitoring the future study, 1975–1994, vol 1. US Department of Health and Human Services, Washington, D.C.
Johnston LD, O’Malley PM, Bachman JG (1997) Monitoring the future study: 1975– Dept Health and Human Services, Washington, D.C.
Jones RT, Benowitz N, Bachman J (1976) Clinical studies of cannabis tolerance and dependence. Ann NY Acad Sci 282:221–239
Jones RT, Benowitz N, Herning RI (1981) Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol 21:143S–152S
Kaymakcalan S (1973) Tolerance to and dependence on cannabis. Bull Narco 25:39–47
Kouri EM, Pope HG Jr, Lukas SE (1998) Changes in aggressive behavior following discontinuation from long-term marijuana use. NIDA Res Monogr Series 178:70
McLaren DS (1976) Nutrition and its disorders, 2nd edn. Churchill Livingstone, New York
Mendelson JH, Mello NK, Lex BW, Bavli S (1984) Marijuana withdrawal syndrome in a woman. Am J Psychiatry 141:1289–1290
Meyer RE, Pillard RC, Shapiro LM, Mirin SM (1971) Administration of marijuana to heavy and casual marijuana users. Am J Psychiatry 128:198–204
404
Nowlan R, Cohen S (1977) Tolerance to marijuana: heart rate and subjective ‘‘high.’’ Clin Pharmacol Ther 22:550–556
Rickles WH Jr, Cohen MJ, Whitaker CA, McIntyre KE (1973) Marijuana induced state-dependent verbal learning. Psychopharmacologia 30:349–354
Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, et al. (1994) SR14176A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350:240–244
Roffman RA, Stephens RS, Simpson EE, Whitaker DL (1988) Treatment of marijuana dependence: preliminary results. J Psychoact Drugs 20:129–137
Stephens RS, Roffman RA, Simpson EE (1993) Adult marijuana users seeking treatment. J Consult Clin Psychol 61:1100–1104
Stephens RS, Roffman RA, Simpson EE (1994) Treating adult marijuana dependence: a test of the relapse prevention model. J Consult Clin Psychol 62:92–99
Tsou K, Patrick SL, Walker JM (1995) Physical withdrawal in rats tolerant to A9_ tetrahydrocannabinol precipitated by a cannabinoid receptor antagonist. Eur J Pharmacol 280:R13–R15
US Department of Health and Human Services (1988) The health consequences of smoking: nicotine addiction: a report of the surgeon general (DHHS Publication No. CDC 88-8406). US Government Printing Office, Washington, D.C.
Wiesbeck GA, Shuckit MA, Kalmijn JA, Tipp JE, Bucholz KK, Smith TL (1996) An evaluation of the history of a marijuana withdrawal syndrome in a large population. Addiction 91:1469–1478
| Index |
| Index |
Last Updated (Monday, 20 December 2010 15:33)












