A detailed characterization of the effects of four cannabinoid agonists on operant lever pressing
Drug Abuse
Psychopharmacology (1998) 137:147–156 © Springer-Verlag 1998
O R I G I NA L I N V E S T I G AT I O N
~~~~~~~D. Carriero · J. Aberman · S.Y. Lin · A. Hill A. Makriyannis · J.D. Salamone
A detailed characterization of the effects of four cannabinoid agonists on operant lever pressing
~~~~~~Received: 16 May 1997 / Final version: 2 December 1997
~~~~~Abstract The present experiments were conducted to investigate the effects of four cannabimimetics on detailed temporal parameters of operant responding. In this study, the behavioral output during performance of a fixed ratio
5 schedule of reinforcement was recorded by a computer program that measured the response initiation time (IT; time interval between the offset of one lever press and the onset of the next) and the response duration (the amount of time that elapses from the onset to the offset of one lever press) of each lever press. ITs were further partitioned into fast responses (IT=0.0–1.0 s), short pauses (IT=1.0–2.5 s), and long pauses (IT>2.5 s). Four cannabimimetic agents were assessed in this study: (R)-methanandamide (AM 356), a hydrolytically stable analog of arachidonylethanolamide, an endogenous ligand for the CB1 receptor; CP-55,940, a potent non-classical synthetic ligand; (-)-A8-tetrahydrocannabinol (A8-THC), an isomer of the naturally occurring A9-THC; and WIN 55,212-2, a synthetic aminoalkylindole. All four of the cannabimimetic drugs tested significantly suppressed operant lever pressing in a dose dependent manner. The rank order of potencies observed in the present study was CP-55,940>>WIN-55,212-2>A8-THC>AM 356, which is consistent with the rank order of affinities for the CB1 receptor shown by these drugs. All of the cannabimimetics substantially increased average IT, and also increased duration time. There was a substantial increase in average length of long pauses, and statistically significant but very small changes in the local rate of responding as measured by the average length of fast ITs. Cannabinoidtreated rats were largely immobile during pauses in responding, and these animals showed several signs of ataxia and catalepsy at the doses that suppressed lever pressing. Together with other data, the present results suggest that CB1 stimulation leads to motor effects that are associated with a suppression of lever pressing.
~~~~~Key words Tetrahydrocannabinol · THC
·
Anandamide · Catalepsy · Motor · Response initiation
D. Carriero · J. Aberman · S.Y. Lin · A. Hill · A. Makriyannis J.D. Salamone (®)
Department of Psychology and School of Pharmacy, University of Connecticut, Storrs, CT 06269-1020, USA~~~~~~~~~~~
Introduction
In 1964, A9-tetrahydrocannabinol (A9-THC) was identified as being the principal active component of marijuana (Gaoni and Mechoulam 1964). This discovery and behavioral tests of newly developed high-potency synthetic cannabinoids led to the search for a specific cannabinoid receptor site. It was reported by Devane et al. (1988) that cannabinoids bind to a specific G-protein coupled receptor, and subsequently the cDNA of the cannabinoid receptor (CB1) was isolated from rat cerebral cortex (Matsuda et al. 1990). This receptor shares over 97% sequence identity with the human CB1 receptor (Gerard et al. 1990). Using the high affinity radiolabeled ligand [3H]CP-55,940, Herkenham et al. (1990) revealed the localization of CB1 receptors throughout the rat brain. Areas with the greatest density of cannabinoid receptors include the substantia nigra pars reticulata, the basal ganglia, the cerebellum, and the hippocampus. The cerebral cortex and the caudate-putamen also contain a moderate number of CB1 receptors.
With these important discoveries has come a renewed interest in determining the behavioral effects of drugs that act upon cannabinoid receptors. Previous researchers have examined the antinociceptive effects of cannabimimetics in various species (Compton et al. 1991; Cook et al. 1995; Hohmann et al. 1995; Lichtman et al. 1995; Martin et al. 1995), spatial memory in rodents (Lichtman et al. 1995), and body temperature (Martin et al. 1981; Compton et al. 1991; Lichtman et al. 1995). Several investigations have focused upon the motor effects of cannabimimetics, and studies have shown that these drugs suppress locomotor activity (Martin et al. 1981; Compton et al. 1991; Crawley et al. 1993; Romero et al. 1995), and induce catalepsy (Gough and Olley 1977; Pertwee et al. 1988; Compton et al. 1991; Prescott et al. 1992; Rodriguez de Fonesca et al. 1994; Lichtman et al. 1995). Although the anatomical basis of the motor effects of can-
148
nabimimetics is uncertain, it has been suggested that basal ganglia mechanisms are involved (Gough and Olley 1977). Within the rat basal ganglia CB1 receptors have been identified on striatal projection neurons (Herkenham
et al. 1991), and cannabimimetics have been shown to attenuate D1-receptor mediated rotational behavior in rodents (Anderson et al. 1995). Collectively, these findings suggest that cannabimimetics produce motor effects by CB1 receptor stimulation in the basal ganglia.
In addition to suppressing locomotion and inducing catalepsy, cannabinoid agonists have been shown to impair lever pressing at moderate/high doses (Boyd et al. 1963; Frankenheim et al. 1971; Martin et al. 1981; Hiltunen et al. 1989; Paule et al. 1991; Smith 1991; Wiley et al. 1995). Typically, the only parameter of response output reported in these studies is response rate. However, recent research has emphasized the use of additional parameters, such as interresponse times, initiation times, and response duration, for characterizing lever pressing performance (Faustman and Fowler 1983; Fowler and Liao 1994). For example, measures of interresponse time and response duration have been used to distinguish between the effects of extinction and dopamine antagonism
or depletion upon lever pressing (Faustman and Fowler
1983; Salamone et al. 1995). It has been suggested that drug-induced increases in response duration could be used as a marker of bradykinesia or catalepsy (Fowler and Liao 1994; Carriero et al. 1997). In recent studies, our laboratory has employed a detailed temporal characterization of operant responding to assess the motor effects of dopamine antagonists (Salamone et al. 1993), dopamine depletion (Sokolowski and Salamone 1994; Salamone et al. 1995; Cousins and Salamone 1996a, b) and cholinomimetics (Carriero et al. 1997). The present
experiments were conducted to investigate the effects of
cannabimimetics on detailed temporal parameters of operant responding in the adult male rat. In some recent studies, detailed analyses of interresponse times (i.e., time from onset of one response to onset of the next response) were performed (Salamone et al. 1993; Sokolowski and Salamone 1994). In the present study, the behavioral output during performance of a fixed ratio 5 (FR5) schedule of reinforcement was recorded by a BASIC computer program that measured the response initiation time (IT) (i.e., the time interval between the offset of one lever press and the onset of the next), and the response duration (DURT) (i.e., the amount of time that elapses from the onset to the offset of one lever press), for each lever press (for review, see Cousins and Salamone 1996a, b). ITs were further partitioned into fast responses (IT=0.0–1.0 s), short pauses (IT=1.0–2.5 s), and long pauses (IT>2.5 s; see Carriero et al. 1997).
The cannabimimetic agents assessed in this study were four agonist drugs: (R)-methanandamide (AM 356), a hydrolytically stable analog of arachidonylethanolamide, an endogenous ligand for the CB1 receptor (Romero et al. 1996); CP-55,940, a potent non-classical synthetic ligand
(for review, see Makriyannis and Rapaka 1990); (-)-A8
tetrahydrocannabinol (z18-THC), an isomer of the natural
ly occurring A9-THC; and (+)-WIN 55,212-2, a synthetic aminoalkylindole (Compton et al. 1992).
Materials and methods
Subjects
Male albino rats (total n=28, Harlan Sprague Dawley, Indianapolis, Ind., USA) were used for these experiments. Animals were group housed in a vivarium with a 12-h light/dark cycle (lights on at 0700 hours) and a constant temperature of 23° C. Average weights at the onset of the study were 320–380 g. Rats were fooddeprived to 85% of their free-feeding body weights and remained food-deprived throughout the study. Water was available ad libitum. These experiments were approved by the university animal care committee that supervises the care and use of animals.
Pharmacological agents
A total of four cannabimimetic drugs were used; AM 356 (MW 361.6) and A8-THC (MW 314.5) were synthesized in the laboratory (A. Makriyannis), while CP-55,940 (MW 376.6) and WIN 55,212-2 (MW 426.0) were kindly donated by Pfizer and Sterling Winthrop, respectively. The cannabimimetics were dissolved in dimethylsulfoxide (DMSO), suspended in Tween 80, and brought to volume with 0.9% physiological saline (1:2:7). Drugs were prepared for IP injection in a volume of 1.0 ml/kg. For a control treatment, rats were injected with the same vehicle used for dissolving and suspending the drug.
Behavioral procedures
All training and testing was conducted in operant chambers (28×23×23 cm). Prior to testing, animals underwent several phases of operant training. Animals were magazine trained for 1 day, and then were trained on a continuous reinforcement schedule (CRF) for 1 week, in which each lever press resulted in one 45 mg pellet (BioServe, Frenchtown, N.J., USA). After CRF training, rats were shifted to a fixed ratio 5 schedule (FR5) in which five lever presses were required to receive one pellet. Rats were trained on the FR5 schedule for a minimum of 2 weeks prior to the initiation of drug testing.
Various behavioral measures were recorded during each operant session by a BASIC computer program written specifically for the FR5 task. In addition to recording the total number of lever presses per rat per session, more detailed parameters of response were recorded. The temporal characteristics of responding were analyzed by partitioning the session time into two components: response initiation time (IT) (the time from the release of one lever press to the initial deflection of the next) and response duration (DURT) (the time during which the lever is deflected). From these, the average IT and DURT were calculated. ITs were further divided into three components: fast responses (2.5 s). Total ITs and average length of ITs (the total divided by the number of initiation times) were calculated for fast responses, short pauses and long pauses. Fast responses were also counted as being within one of eight time bins, each representing an interval of 125 ms. This further analysis was conducted because most of the ITs that occurred under baseline or control conditions were within the fast response category, and the analysis of 125-ms bins allowed for a detailed characterization of these fast responses. Duration times were also sorted into 125-ms time bins.
Experiments
Rats were trained to perform the operant task as described above. Daily baseline training sessions continued on those days that were not drug treatment days until the experiment was completed. Within
149
each experiment a repeated measures design was used, with each rat received all drug treatments (one injection per week) in a randomly varied order. The 30-min operant test session was initiated 10 min after drug administration (the 10-min interval was based upon pilot data). In each of the four experiments, there were six drug treatments: AM356 (vehicle, 0.625, 1.25, 2.5, 5.0, and 10.0 mg/kg), CP-55,940 (vehicle, 0.031, 0.0625, 0.125, 0.25, and 0.5 mg/kg), A8THC (vehicle, 0.625, 1.25, 2.5, 5.0, and 10.0 mg/kg), and WIN 55,212-2 (vehicle, 0.156, 0.3125, 0.625, 1.25, and 2.5 mg/kg).
Data analysis
Repeated measures analysis of variance (ANOVA) was used to analyze the total number of lever presses. Raw data were log transformed prior to conducting statistical tests if the homogeneity of variance assumption for ANOVA was violated. Planned comparisons were used to test for differences between each drug dose and the vehicle-control condition (Keppel 1982). Additional parameters of responding (average IT, average DURT, the number and average length of fast responses, short pauses and long pauses) were analyzed in two ways. Data on the various behavioral parameters were analyzed by linear regression methods, in which the relation between dose and behavioral response was determined; a significant F value from the regression analysis (i.e., General Linear Model, Systat 5.0) was interpreted as a significant effect of drug treatment. In addition, behavioral data were analyzed using repeated measures t-tests, comparing each drug dose to the vehicle-control as a planned comparison (Keppel 1982). Each dose was compared with control separately because at higher doses of several drugs several animals had zero lever presses. Therefore all measures of responding could not be obtained for all animals at all doses. Consistent with the method described by Keppel (1982), the analyses of each drug dose to control involved five planned comparisons (significance level=0.05; approximate familywise error=0.20), with the exception of CP-55,940 which had four comparisons and a familywise error of 0.20. For graphic depiction of the IT and DURT bins, data in each bin were expressed as a proportion of the total number of fast responses and fast durations, respectively, so that the relative frequency distributions of fast responses and durations could be examined. This method was employed to adjust for treatment differences in the total number of fast responses and durations in order for the relative frequency distributions of fast ITs and DURTs to be examined (see Salamone et al. 1993; Cousins and Salamone 1996; Carriero et al. 1998). The distributions of response ITs and DURTs were analyzed by testing for significant differences between the proportions obtained in each bin (Bruning and Kintz 1987), with each dose being tested against the vehicle-control.
Because of the large number of comparisons for AM356, A8-THC, and WIN 55,212-2 (5 comparisons×8 bins=40), the significance level was set at P=0.005 (familywise error of 0.20 divided by 40). Similarly, the significance level for CP-55,940 was set at P=0.006 (familywise error of 0.20 divided by 32; 4 comparisons×8 bins).
Results
Experiment 1: effect of AM 356 administration on lever pressing
The effect of AM 356 on the total number of lever presses is shown in Fig. 1. AM 356 produced a decrease in the
Fig. 1 Mean (±SEM) number of lever presses under control conditions (0 mg/kg) and various doses of cannabinoid agonists (doses expressed on a log scale). Note that the dose-response curves for each drug are roughly parallel. See text for exact doses used for each study. • AM 356, ∎ CP-55,940, A A8-THC, V WIN 55,212-2&/fig.c:
1800
0.0 0.1 1.0 10.0
Dose (mg/kg)
1600 -
1400 -
• 1200 - U) U) i 1000 -
CL
L 800 -
m
J 600 -
400 - 200 -
L
-~ AM 356
-~ - DELTA 8 THC
t CP-55,940 -y- WIN 55,212-2
I
Table 1 Average DURT and average IT. Absolute number, percentage and length of fast responses, short pauses and long pauses. Means (±SEM) for each parameter are shown&/tbl.c:&tbl.b:
|
Parameter |
Dose AM356 (mg/kg) |
|||||
|
Vehicle (n=6) |
0.625 (n=6) |
1.25 (n=6) |
2.5 (n=6) |
5.0 (n=6) |
10.0 (n=6) |
|
|
Average DURT |
0.25 (0.01) |
0.248 (0.01) |
0.258 (0.01) |
0.257 (0.01) |
0.293 (0.02) |
0.457* (0.06)# |
|
Average IT |
0.966 (0.09) |
1.12 (0.20) |
0.934 (0.05) |
1.33* (0.17) |
1.48 (0.21) |
10.04* (3.08)# |
|
Percent fast responses |
76.36 (0.88) |
75.47 (1.84) |
77.36 (1.04) |
74.76 (2.78) |
72.72 (2.80) |
68.59 (4.12)# |
|
Percent short pauses |
16.70 (0.92) |
16.34 (0.76) |
14.70 (0.81) |
14.54* (0.91) |
15.55 (2.22) |
17.57 (2.12) |
|
Percent long pauses |
6.45 (1.15) |
8.19 (1.86) |
7.94 (1.04) |
10.69 (1.92) |
11.72 (1.64) |
13.84 (2.97)# |
|
average length of |
0.17 (0.02) |
0.17 (0.02) |
0.16 (0.02) |
0.19 (0.03) |
0.19 (0.03) |
0.24* (0.02)# |
|
fast responses (s) Average length of |
1.69 (0.04) |
1.67 (0.04) |
1.72 (0.03) |
1.71 (0.02) |
1.64 (0.01) |
1.65 (0.04) |
|
short pauses (s) Average length of |
8.60 (1.40) |
10.36 (3.99) |
7.36 (0.63) |
8.95 (0.68) |
8.82 (0.97) |
74.57* (30.43)# |
|
long pauses (s) |
||||||
* P<0.05, different from vehicle # P<0.05, regression analysis&/tbl.b:
150
AM-356
1.0
0.9
N
G 0.8
a+
0.7
L
Q • 0.6
4
0.5
C
0.4
0.3
O.
0
0.2
d
0.1
0.0
Duration Time Bin (msec)
VEHICLE
0.625 mg/kg = 1.25 mg/kg
2.5 mg/kg -- 5.0 mglkg IIIIIIIIIII 10.0 mg/kg
CP 55,940
VEHICLE 0.031 mg/kg ® 0.0625 mg/kg
0.125 mg/kg 0.25 mg/kg
1.0
4
0.5
C
0.4
t
0.3
O.
0
0.2
I1
0.1
0.0
I I
IiI ?I 11"Ilm -11
Ill 11 1
WLI .'al ...gill ..1111
125 250 375 500 625 750 875 1000
Duration Time Bin (msec)
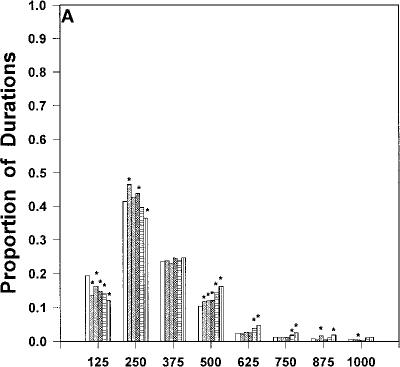
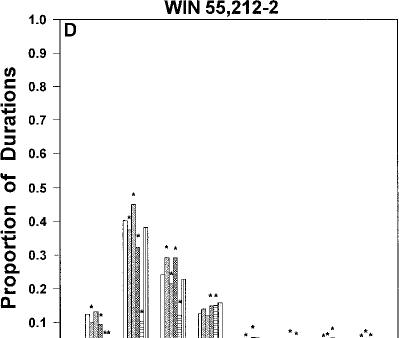
Fig. 2 A–D Relative frequency distributions of response duration times (expressed as proportion of total within each bin) for injections of vehicle and each dose of cannabinoid agonist. A AM 356.
B CP 55,940. C A8-THC. D WIN 55,212-2. * P<0.05, different from control in that time bin&/fig.c:
number of lever presses [F(5, 25)=13.0, P<0.0001]. Planned comparisons indicated that only the high dose of 10.0 mg/kg differed from vehicle (P<0.05). Table 1 shows the effect of AM 356 on the average response 356 produced a significant increase in response duration at the high dose of 10.0 mg/kg [t(5)=4.0, P<0.05]. The relative distribution of response duration times within each of the eight 125-ms time bins was analyzed (Fig. 2a). AM 356 produced a significant decrease in fast response DURTs (i.e., 0–125 ms) and a significant
125 250 375 500 625 750 875 1000
Duration Time Bin (msec)
VEHICLE ® 0.156 mg/kg = 0.3125 mg/kg 0.625 mglkg t -1 1.25 mglkg IM 2.5 mglkg
increase in the proportion of longer response DURTs (e.g., 375–500 ms).
AM 356 administration significantly increased the average initiation time (Table 1). Table 1 also depicts the effect of AM 356 administration on the breakdown components of response initiation times. AM356 significantly decreased the number of responses in all three categories (i.e., fast, short and long pauses; data not shown). In addition, AM356 significantly decreased the percentage of fast responses and increased the percentage of long pauses. AM356 produced a statistically significant but small increase (i.e., about 50%) in the average length of fast responses, which indicates a slight decrease in the local rate of responding. In addition, AM356 produced a substantial increase (about 900%) in the average length of long pauses. Figure 3a shows the relative distribution of response initia-
VEHICLE
® 0.625 mg/kg 1.25 mg/kg
2.5 mg/kg
I— 7
5.0 mg/kg 11] 10.0 mglkg


1.0
AM-356
1.0
CP-55,940
A
0.9 -
08
0.7 -
B
P
0.6 -
0.5 -
0.4 -
0.3 -
0.2 -
4
0.5 -
C
0.4 -
r L
• 0.3 - CL
L. 0.2 -
I
0.1 -
125 250 375 500 625 750 875 1000
IL
0.1 -
0.0
0.0
Initiation Time Bin (msec)
,L _7
125 250 375 500 625 750 875 1000
Initiation Time Bin (msec)
VEHICLE
1 0.625 mg/kg 1 1.25 mg/kg
2.5 mg/kg I J 5.0 mglkg M 10.0 mglkg
VEHICLE 0.031 mglkg 0.0625 mglkg
0.125 mg/kg E 0.25 mg/kg
1.0
A8-THC
WIN 55,212-2
4
0.5
C
0.4
a+ L
• 0.3 L. 0.2
I
a
0.1
0.0
.®ks 1®1111 u 1111 -e:.' :e .vacs.
125 250 375
500 625 750 875 1000
VEHICLE 0.625 mg/kg 1.25 mglkg
2.5 mg/kg I 5.0 mg/kg UM 10.0 mglkg
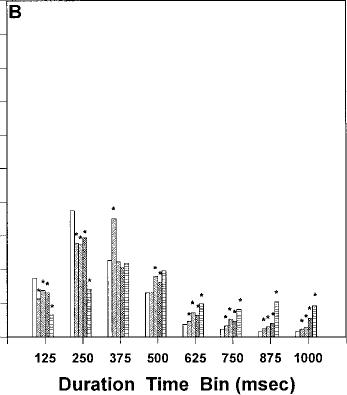
Fig. 3 A–D Relative frequency distributions of response initiation times (expressed as proportion of total within each bin) for injections of vehicle and each dose of cannabinoid agonist. A AM 356.
B CP 55,940. C A8-THC. D WIN 55,212-2. * P<0.05, different from control in that time bin&/fig.c:
tion times within each of the eight 125-ms time bins. Although the basic distribution of fast initiation times was similar for each drug treatment condition (e.g., mode in the first time bin), AM 356 produced small but significant alterations in the proportion of responses in some time bins. Similar effects on initiation time bins were induced by the other three cannabinoids (see below). In addition, it was observed for AM 356 and the other three cannabimimetics that drug-treated rats consumed all the operant pellets in the food dish, even if the rats showed suppressed responding.
Inititation Time Bin (msec)
Experiment 2: effect of CP-55,940 administration on lever pressing
The effect of CP-55,940 on the total number of lever presses are shown in Fig. 1. CP-55,940 produced a potent dose-related decrease in the total number of lever presses [F(4, 28)=13.193, P<0.0001]. Planned comparisons indicated that the 0.125 mg/kg, and 0.25 mg/kg and 0.5 mg/kg doses significantly differed from vehicle (P<0.05). Table 2 shows the effect of CP-55,940 on the average response duration (only one rat treated with 0.5 mg/kg showed any responses, so this dose was excluded from any additional analyses). CP-55,940 produced a significant increase in response duration at the following doses: 0.031 mg/kg [t(7)=2.4, P<0.05]; 0.0625 mg/kg [t(6)=4.3, P<0.01]; 0.125 mg/kg [t(7)=3.8, P<0.01]; and
I I
VEHICLE
0.156 mglkg
0.625 mg/kg 1.25 mg/kg
111111111111
0.3125 mg/kg 2.5 mg/kg

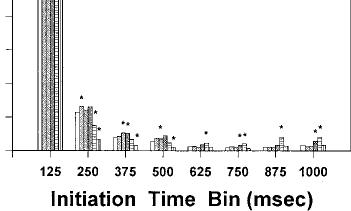


Table 2 Average DURT and average IT. Absolute number, percentage and length of fast responses, short pauses and long pauses. Means (±SEM) for each parameter are shown&/tbl.c:&tbl.b:
|
Parameter |
Dose CP-55,940 (mg/kg) |
|||||
|
Vehicle (n=8) |
0.031 (n=8) |
0.0625 (n=7) |
0.125 (n=8) |
0.25 (n=6) |
||
|
Average DURT |
0.31 (0.02) |
0.364* (0.03) |
0.454* (0.05) |
0.645* (0.11) |
1.16* (0.24)# |
|
|
Average IT |
0.923 (0.06) |
1.08 (0.10) |
2.02* (0.48) |
11.55* (4.0) |
140.74* (92.7)# |
|
|
Percent fast responses |
74.54 (2.47) |
74.54 (2.10) |
63.78 (10.10) |
63.52* (6.45) |
43.23 |
(13.12) |
|
Percent short pauses |
17.10 (2.34) |
14.46 (1.82) |
12.10 (3.22) |
18.69 (4.20) |
11.43 |
(4.55) |
|
Percent long pauses |
8.36 (1.45) |
11.00 (1.87) |
11.62 (2.84) |
17.79* (3.25) |
20.34 |
(11.65) |
|
Average length of |
0.18 (0.02) |
0.17 (0.01) |
0.17 (0.02) |
0.22 (0.04) |
0.21 |
(0.04) |
|
fast responses (s) Average length of |
1.73 (0.04) |
1.71 (0.03) |
1.64 (0.03) |
1.73 |
(0.15) |
|
|
1.59* (0.03) |
||||||
|
short pauses (s) Average length of |
6.71 (1.08) |
7.38 (0.98) |
13.55* (2.60) |
71.53* (25.04) |
531.9* |
(159.0)# |
|
long pauses (s) |
||||||
* P<0.05, different from vehicle # P<0.05, regression analysis&/tbl.b:
0.25 mg/kg [t(5)=4.7, P<0.01]. The relative distribution of response duration times within each of the eight 125ms time bins was analyzed and are displayed in Fig. 2b. CP-55,940 significantly suppressed the relative number of response durations in the fastest bin (i.e., 0–125 ms), and increased the proportion of durations in slower bin categories (i.e., 375–1000 ms). In addition, the high dose of CP-55,940 shifted the modal duration from bin 2 (125–250 ms) to bins 3 and 4 (250–500 ms).
CP-55,940 produced a significant increase in the average response initiation time (Table 2). In Table 2, the effect of CP-55,940 administration on the breakdown components of response initiation times can be seen. CP55,940 significantly decreased the number of responses in all three categories (i.e., fast, short and long pauses; data not shown). In addition, CP-55,940 significantly decreased the percentage of fast responses in a dose-related manner, and increased the percentage of long pauses at the second highest dose (0.125 mg/kg). CP-55,940 produced no statistically significant change in the average length of fast responses. Nevertheless, CP-55,940 did produce a substantial increase (about 80-fold) in the average length of long pauses. Figure 3b shows the relative distribution of response initiation times within each of the eight 125-ms time bins.
Experiment 3: effect of A8-THC administration on lever pressing
The effect of A8-THC on the total number of lever presses is shown in Fig. 1. A8-THC produced a dose-related decrease in the number of lever presses [F(5, 35)=7.7, P<0.0001]. Planned comparisons revealed that the doses of 5.0 mg/kg and 10.0 mg/kg A8-THC significantly differed from vehicle (P<0.05). Table 3 shows the effect of A8-THC on the average response duration. A8-THC did not produce a significant increase in duration at any
dose. However, a linear regression analysis demonstrated that, across all drug conditions, there was a significant linear relation between dose and duration (P<0.05), with the significant positive slope indicating that increasing drug dose led to increased response durations. The relative distribution of response duration times within each of the eight 125-ms time bins was analyzed and depicted in Fig. 2c. The highest dose of A8-THC produced a significant increase in the relative number of response durations in bins 4, 5 and 6 (375–750 ms).
Administration of A8-THC significantly increased the average initiation time (Table 3). The effects of this drug on the various components of the response initiation time also are shown in Table 3. A8-THC significantly decreased the number of responses in all three categories (i.e., fast, short and long pauses; data not shown). A8THC significantly decreased the percentage of fast responses, and had an effect on the percentage of long pauses that approached statistical significance (P=0.57). A8-THC produced a statistically significant but small increase (i.e., about 50%) in the average length of fast responses, which indicates a slight decrease in the local rate of responding. In addition, this drug produced a substantial increase (about 50-fold) in the average length of long pauses. Figure 3c shows the relative distribution of response initiation times within each of the eight 125-ms time bins.
Experiment 4: effects of WIN 55,212-2 administration on lever pressing
The effect of WIN 55,212-2 on the total number of lever presses is shown in Fig. 1. WIN 55,212-2 produced a significant decrease in the number of lever presses [F(5, 25)=22.0, P<0.0001]. Planned comparisons revealed that the doses of 1.25 mg/kg and 2.5 mg/kg dose differed significantly from vehicle (P<0.05). Table 4 shows the ef-
Table 3 Average DURT and average IT. Absolute number, percentage and length of fast responses, short pauses and long pauses. Means (±SEM) for each parameter are shown&/tbl.c:&tbl.b:
|
Parameter |
Dose A8-THC (mg/kg) |
||||||
|
Vehicle (n=8) |
0.625 (n=8) |
1.25 (n=8) |
2.5 (n=8) |
5.0 (n=7) |
10.0 (n=5) |
||
|
Average DURT |
0.319 (0.02) |
0.293 (0.02) |
0.30 (0.02) |
0.343 (0.04) |
0.667 (0.19) |
0.942 (0.31)# |
|
|
Average IT |
1.06 (0.06) |
45.97 (44.83) |
1.85* (0.38) |
3.29* (0.45) |
95.30* (83.96) |
96.44 |
(88.03)# |
|
Percent fast responses |
77.57 (1.84) |
69.81 (10.04) |
74.93 (1.75) |
74.05 (2.38) |
54.33 (9.94) |
53.03 |
(15.74)# |
|
Percent short pauses |
11.72 (1.28) |
14.44 (5.34) |
13.62 (2.01) |
15.38 (1.99) |
25.28* (5.52) |
14.48 |
(5.28)# |
|
Percent long pauses |
10.71 (1.52) |
15.74 (5.05) |
11.44 (1.61) |
10.57 (1.88) |
20.39 (5.22) |
32.49 |
(17.64) |
|
Average length of |
0.14 (0.02) |
0.14 (0.02) |
0.14 (0.01) |
0.16 (0.01) |
0.21* (0.01) |
0.18 |
(0.08)# |
|
fast responses (s) Average length of |
1.76 (0.05) |
1.66 (0.05) |
1.69 (0.03) |
1.72 (0.11) |
1.53 |
(0.12)# |
|
|
1.57* (0.06) |
|||||||
|
short pauses (s) Average length of |
7.92 (1.1) |
119.3 (111.2) |
15.86 (4.8) |
34.90* (9.1) |
318.48* (246.5) |
164.6* |
(110.0)# |
|
long pauses (s) |
|||||||
* P<0.05, different from vehicle # P<0.05, regression analysis&/tbl.b:
Table 4 Average DURT and average IT. Absolute number, percentage and length of fast responses, short pauses and long pauses. Means (±SEM) for each parameter are shown&/tbl.c:&tbl.b:
|
Parameter |
Dose WIN 55,212-2 (mg/kg) |
|||||
|
Vehicle (n=6) |
0.156 (n=6) |
0.3125 (n=6) |
0.625 (n=6) |
1.25 (n=6) |
2.50 (n=3) |
|
|
Average DURT |
0.348 (0.05) |
0.342 (0.04) |
0.322 (0.06) |
0.50 (0.10) |
0.867* (0.11) |
0.427 (0.14)# |
|
Average IT |
0.883 (0.10) |
0.933 (0.12) |
1.02* (0.15) |
4.17* (2.41) |
107.34* (98.4) |
450.59 (449.33)# |
|
Percent fast responses |
83.23 (2.0) |
81.81 (2.4) |
80.15 (3.4) |
72.29* (3.7) |
55.94 (12.7) |
30.1 (30.1)* |
|
Percent short pauses |
9.23 (1.0) |
9.77 (1.3) |
10.04 (2.1) |
10.98 (2.0) |
13.86 (5.6) |
1.49 (1.49)# |
|
Percent long pauses |
7.54 (1.3) |
8.43 (1.6) |
9.81 (1.8) |
16.73* (2.5) |
30.20* (14.1) |
68.42 (31.6)# |
|
Average length of |
0.11 (0.01) |
0.11 (0.01) |
0.123 (0.01) |
0.16* (0.01) |
0.18 (0.03) |
0.13 (n/a)# |
|
fast responses (s) Average length of |
1.70 (0.05) |
1.65 (0.04) |
1.66 (0.04) |
1.63 (0.05) |
1.64 (0.05) |
1.58 (n/a) |
|
short pauses (s) Average length of |
9.13 (1.0) |
9.03 (2.1) |
8.25 (1.2) |
18.59* (8.8) |
195.67 (142.1) |
1206.59 (593.0)# |
|
long pauses (s) |
||||||
* P<0.05, different from vehicle
# P<0.05, regression analysis
n/a=only one animal performed fast responses or short pauses
fect of WIN 55,212-2 on the average response duration. WIN 55,212-2 produced a significant increase in response duration at 1.25 mg/kg [t(5)=5.0, P<0.01]. The relative distribution of response duration times within each of the eight 125-ms time bins was analyzed (Fig. 2d). WIN 55,212-2 produced a significant decrease in the fast response DURTs (0–125 ms) and a significant increase in the slow response DURTs.
Injections of WIN 55,212-2 significantly increased the average initiation time (Table 4). This table also
shows the effect of this drug on the various components of the response initiation time. WIN 55,212-2 significantly decreased the number of responses in all three categories (i.e., fast, short and long pauses; data not shown).
WIN 55,212-2 significantly decreased the percentage of fast responses, and significantly increased the percentage
of long pauses. WIN 55,212-2 produced a statistically significant but small increase in the average length of fast responses, which indicates a slight decrease in the local rate of responding. In addition, this drug produced a very large increase in the average length of long pauses. Figure 3d shows the relative distribution of response initiation times within each of the eight 125-ms time bins.
Discussion
All four of the cannabimimetic drugs tested significantly suppressed operant lever pressing in a dose dependent manner. The present results suggest that the suppression of lever pressing was related to an action on the CB1 can-
nabinoid receptor. The rank order of potency observed in the present study was CP-55,940>>WIN-55,212-2>A8THC>AM 356, which is consistent with the rank order of affinities for the CB1 receptor shown by these drugs (Compton et al. 1993). In a parallel study, it has been demonstrated that the CB1 receptor antagonists AM 251 (1.0–4.0 mg/kg) and SR141716A (1.0 mg/kg) were able partially to reverse the lever pressing deficits produced by 0.125 mg/kg of the high-potency CB1 agonist CP-55,940 (Aberman et al., submitted). Taken together, these results indicate that stimulation of CB1 receptors can result in a suppression of operant lever pressing. The fact that AM 356, a stable analogue of the endogenous cannabimimetic ligand, suppressed lever pressing in a manner similar to the other cannabimimetics suggests that endogenous cannabinoid compounds can have behavioral effects via stimulation of the CB1 receptor.
The detailed behavioral methods used in the present study allowed for a precise characterization of the pattern of lever pressing suppression shown by cannabinoid agonists. All four of the cannabimimetic drugs showed a similar pattern of effects. Average response initiation time was substantially increased in a dose-related manner. In addition, there were significant increases in the relative number and average length of long pauses, and significant increases in response duration. These effects are similar to those previously reported for ventrolateral striatal dopamine depletions (Cousins and Salamone 1996a, b), the dopamine antagonist haloperidol (Carriero 1997), and the anticholinesterase tacrine (Carriero et al. 1997). However, in those other studies cholinergic stimulation and dopamine antagonism also substantially affected the local rate of response initiation within bursts (e.g., 2.5-fold increases in average length of fast ITs, up to 400 ms). In contrast, despite the fact that total numbers of responses were severely affected by cannabimimetic drugs, the local rate of response initiation (i.e., average length of fast responses) was not significantly altered by CP 55-940, and was only minimally affected by the other three cannabinoids (up to 180–240 ms). Although statistically significant effects on the distribution of response initiation times were observed after cannabimimetic injection, these effects were small and inconsistent compared to similar studies of tacrine, haloperidol and pentobarbital (Carriero 1997; Carriero et al. 1997). For example, haloperidol and tacrine produced large (i.e., 15–50%) dose-related reductions in the relative number of fast ITs in the first time bin. In the present study, CP 55,940 and A8-THC did not reduce this measure in a consistent, dose-related manner, while AM-356 and WIN 55,212-2 produced only modest effects on this measure (i.e., less than 15% reductions). In summary, other studies with identical behavioral methods have shown that the decrease in responding produced by pentobarbital is characterized largely by a substantial decrease in the local rate of response initiation (i.e., increase in the average length of fast responses, loss of 0–125 ms ITs), while tacrine and haloperidol lead to substantial decreases in the local rate of response initiation
as well as large increases in the average length of long pauses (Carriero 1997; Carriero et al. 1997). In contrast, cannabimimetic-treated rats show very long pauses in responding, yet during bursts of responding (i.e., fast ITs) the local rate of response initiation is relatively normal. These data suggest that various response-suppressing drugs can be distinguished by the detailed temporal patterns of their effects, and indicate that the suppression of lever pressing produced by cannabimimetics was similar, but not identical, to that produced by neuroleptics or cholinomimetics.
The precise mechanisms underlying the cannabimimetic-induced suppression of lever pressing are unclear. However, several lines of evidence indicate that cannabimimetics suppressed lever pressing due to effects on motor function. Cannabimimetic-treated rats were observed in the operant chambers during periods of non-responding, and it was noted that these rats were akinetic. The substantial increase in average length of long pauses and the presence of akinesia during these pauses are consistent with previous reports that cannabimimetics increase the amount of time spent immobile (Romero et al. 1995, 1996). In the present study, cannabimimetic-treated rats also showed signs of sedation, ataxia, splayed hindlimbs and poor forelimb placement (i.e., forelimbs placed through the grid floor) during periods of non-responding. These observations of ataxia are consistent with the classic work of Adams (e.g., Adams et al. 1941), who used the dog ataxia test to assess structure-activity relations of various cannabinoids. A few of the cannabimimetictreated rats were tested for catalepsy after the operant sessions, and these rats all showed cataleptic immobility. In addition, the cannabimimetic drugs all produced doserelated increases in response duration. This measure is thought to be related to bradykinesia or catalepsy (Liao and Fowler 1990; Fowler and Liao 1994; Carriero et al. 1997). It has previously been noted that catalepsy occurs concomitantly with the suppression of lever pressing in cannabimimetic-treated rats (Martin et al. 1991). These observations are consistent with previous studies showing that cannabimimetics induce motor deficits at moderate/high doses (Adams et al. 1941; Martin et al. 1981; Pertwee et al. 1988, 1991; Compton et al. 1991; Prescott et al. 1992; Crawley et al. 1993; Rodriguez de Fonesca et al. 1994; Romero et al. 1995; Lichtman et al. 1995).
Although the anatomical basis of these effects is uncertain, considerable evidence indicates that there is a high concentration of CB1 receptors in basal ganglia areas. It has been suggested that cannabimimetics modulate the GABAergic activity of striatonigral neurons (Richter and Loscher 1994; Miller and Walker 1995) and that they may modulate dopamine transmission in striatum (Navarro et al. 1993; Souilhac et al. 1995). Studies in which A9-THC is administered via intrastriatal or intrapallidal injection have shown that A9-THC can induce catalepsy in a dose dependent manner at these sites (Gough and Olley 1978; Pertwee et al. 1991). Future investigations should explore the anatomical basis of the lever pressing suppression induced by cannabinoid agonists.
References
Adams R, Lowew, S, Jelinek, C, Wolff H (1941) Tetrahydrocannabinol homologs with marihuana activity. J Acad Chem Soc 63: 1971–1978
Anderson LA, Anderson JJ, Chase TN, Walters JR (1995) The cannabinoid agonists WIN 55,212-2 and CP 55,940 attenuate rotational behavior induced by a dopamine D1 but not a D2 agonist in rats with unilateral lesions of the nigrostriatal pathway. Brain Res 691: 106–114
Boyd ES, Hutchinson ED, Gardner LC, Merrit DA (1963) Effects of tetrahydrocannabinol and other drugs on operant behavior in rats. Arch Int Pharmacodyn 144: 533–554
Bruning JL, Kintz BL (1987) Computational handbook of statistics. Scott, Foresman and Co., Glenview, Ill.
Carriero DL (1997) A detailed characterization of the response-suppressing effects of cannabinoid, neuroleptic, sedative and cholinomimetic drugs. Masters Thesis, University of Connecticut
Carriero DL, Outslay G, Mayorga AJ, Aberman JE, Gianutsos G, Salamone JD (1997) Motor dysfunction produced by tacrine administration in rats, Pharmacol Biochem Behav 58: 851–858
Compton DR, Johnson MR, Melvin LS, Martin BR (1991) Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharmacol Exp Ther 260: 201–209
Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR (1992) Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from A9-tetrahydrocannabinol. J Pharmacol Exp Ther 263: 1118–1126
Compton DR, Rice KC, de Costa DR, Razdan RK, Melvin LS, Johnson MR, Martin BR (1993) Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther 265: 218–226
Cook SA, Welch SP, Lichtman AH, Martin BR (1995) Evaluation of cAMP involvement in cannabinoid-induced antinociception. Life Sci 56: 2049–2056
Cousins MS, Salamone JD (1996a) Involvement of ventrolateral striatum dopamine in movement initiation and execution: a microdialysis and behavioral investigation. Neurosci 70: 849–859
Cousins MS, Salamone JD (1996b) Skilled motor deficits in rats induced by ventrolateral striatal dopamine depletions: behavioral and pharmacological characterization. Brain Res 732: 186–194
Devane WA, Dysarz FA III, Johnson MR, Melvin LS, Howlett AC (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34: 605–613
Faustman WO, Fowler SC (1981) Use of operant response duration to distinguish between the effects of haloperidol and nonreward. Pharmacol Biochem Behav 15: 327–329
Fowler SC, Liou JR (1994) Microcatalepsy and disruption of forelimb usage during operant behavior: differences between dopamine D1 (SCH-23390) and D2 (raclopride) antagonists. Psychopharmacology 115: 24–30
Frankenheim JM, McMillan DE, Harris LS (1971) Effects of l-A8trans-tetrahydrocannabinol and cannabinol on schedule-controlled behavior of pigeons and rats. J Pharmacol Exp Ther 178: 241–252
Gaoni Y, Mechoulam R (1964) Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc 86: 1646–1648
Gerard WA, Mollereau C, Vassart G, Parmentier M (1990) Nucleotide sequence of human cannabinoid receptor cDNA. Nucleic Acids Res 18: 7142
Gough AL, Olley JE (1977) A9-Tetrahydrocannabinol and the extrapyramidal system. Psychopharmacology 54: 87–99
Gough AL, Olley JE (1978) Catalepsy induced by intrastriatal injections of A9-THC and 11-OH-A9-THC in the rat. Neuropharmacology 17: 137–144
Herkenham M, Lynn MD, Little AB, Johnson MK, Melvin LS, de Costa BR (1990) Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA 87: 1932–1936
Herkenham M, Lynn AB, de Costa BR, Richfield EK (1991) Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res 547: 267–274
Hiltunen AJ, Jarbe TUC, Kamkar MR, Archer T (1989) Behavior in rats maintained by low differential reinforcement rate: effects of A1-tetrahydrocannabinol, cannabinol and cannabidiol, alone and in combination. Neuropharmacology 28: 183–189
Hohmann AG, Martin WJ, Tsou K, Walker JM (1995) Inhibition of noxious stimulus-evoked activity of spinal cord dorsal horn neurons by the cannabinoid WIN 55,212-2. Life Sci 56: 2111–2118
Keppel G (1982) Design and analysis: a researchers handbook. Prentice-Hall, Englewood Cliffs, N.J.
Liao RM, Fowler SC (1990) Haloperidol produces within-session increments in operant response duration in rats. Pharmacol Biochem Behav 36: 199–201
Lichtman AH, Dimen KR, Martin BR (1995) Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology 119: 282–290
Makriyannis A, Rapaka RS (1990) Minireview: the molecular basis of cannabinoid activity. Life Sci 47: 2173–2184
Martin BR, Balster RL, Razdan RK, Harris LS, Dewey WL (1981) Behavioral comparisons of the stereoisomers of tetrahydrocannabinols. Life Sci 29: 565–574
Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ (1991) Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav 40: 471–478
Martin WJ, Patrick SL, Coffin PO, Tsou K, Walker JM (1995) An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sci 56: 2103–2109
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346: 561–564
Miller AS, Walker JM (1995) Effects of a cannabinoid on spontaneous and evoked neuronal activity in the substantia nigra pars reticulata. Eur J Pharmacol 279: 179–185
Navarro M, Fernandez-Ruiz JJ, De Miguel R, Hernandez ML, Cebeira M, Ramos JA (1993) Motor disturbances induced by an acute dose of A9-tetrahydrocannabinol: possible involvement of nigrostriatal dopaminergic alterations. Pharmacol Biochem Behav 45: 291–298
Paule MG, Allen RR, Bailey JR, Scallet AC, Ali SF, Brown RM, Slikker W Jr (1991) Chronic marijuana smoke exposure in the rhesus monkey II: effects on progressive ratio and conditioned position responding. J Pharmacol Exp Ther 260: 210–222
Pertwee RG, Greentree SG, Swift PA (1988) Drugs which stimulate or facilitate central GABAergic transmission interact synergistically with A9-tetrahydrocannabinol to produce marked catalepsy in mice. Neuropharmacology 27: 1265–1270
Pertwee RG, Browne SE, Ross TM, Stretton CD (1991) An investigation of the involvement of GABA in certain pharmacological effects of A9-tetrahydrocannabinol. Pharmacol Biochem Behav 40: 581–585
Prescott WR, Gold LH, Martin BR (1992) Evidence for separate neuronal mechanisms for the discriminative stimulus and catalepsy induced by A9-THC in the rat. Psychopharmacology 107: 117–124
Richter A, Loscher W (1994) (+)-WIN 55,212-2, a novel cannabinoid receptor agonist, exerts antidystonic effects in mutant dystonic hamsters. Eur J Pharmacol 264: 371–377
Rodriguez de Fonseca F, Calderon JLM, Mechoulam R, Navarro M (1994) Repeated stimulation of D1 dopamine receptors enhances (-)-11-hydroxy-A8-tetrahydrocannabinol-dimethyl heptyl-induced catalepsy in male rats. Neuropharm Neurotox 5: 761–765
Romero J, Garcia L,Cebeira M, Zadrozny D, Fernandez-Ruiz JJ, Ramos JA (1995) The endogenous cannabinoid receptor ligand, anandamide, inhibits the motor behavior: role of nigrostriatal dopaminergic neurons. Life Sci 56: 2033–2040
Romero J, Garcia-Palomero E, Lin SY, Ramos JA, Makriyannis A, Fernandez-Ruiz JJ (1996) Extrapyramidal side effects of methanandamide, an analog of anandamide, the endogenous CB1 receptor ligand. Life Sci 58: 1249–1257
Salamone JD, Kurth P, McCullough LD, Sokolowski JD (1993a) The effects of nucleus accumbens dopamine depletions on continuously reinforced operant responding: contrasts with the effects of extinction. Pharmacol Biochem Behav 50: 437–443
Salamone JD, Kurth PA, McCullough LD, Sokolowski JD (1993b) The role of brain dopamine in response initiation: effects of haloperidol and regionally specific dopamine depletions on the local rate of instrumental responding. Brain Res 628: 218–226
Sokolowski JD, Salamone JD (1994) Effects of dopamine depletions in the medial prefrontal cortex on DRL performance and motor activity in the rat. Brain Res 642: 20–28
Souilhac J, Poncelet M, Carmona-Rinaldi M, Le Fur G, Soubrie P (1995) Intrastriatal injection of cannabinoid receptor agonists induced turning behavior in mice. Pharmacol Biochem Behav 51: 3–7
Smith JB (1991) Situational specificity of tolerance to decreased operant responding by morphine and l-nantadol. Psychopharmacology 103: 115–120
Wiley JL, Barrett RL, Lowe J, Balster RL, Martin BR (1995) Discriminative stimulus effects of CP 55,940 and structurally dissimilar cannabinoids in rats. Neuropharmacology 34: 669– 676
| Index |
| Index |
Last Updated (Monday, 20 December 2010 15:16)












