|
The Discovery of LSD and Subsequent Investigations on Naturally Occurring Hallucinogens |
 |
 |
 |
|
Written by Albert Hofmann
|
|
Thursday, 07 January 2010 20:40 |
The Discovery of LSD and Subsequent Investigations
on Naturally Occurring Hallucinogens
Albert Hofmann, PhD.
Director of Research, Department of Natural Products, Sandoz Ltd., Basel, Switzerland.
Chapter 7 of Discoveries in Biological Psychiatry,
Frank J. Ayd, Jr. & Barry Blackwell, eds.,
©J.B. Lippincott Company, 1970

IT IS OFTEN STATED in the literature that LSD was discovered by chance. The following account will show that LSD was not the fruit of a chance discovery, but the outcome of a more complex process that had its beginnings in a definite concept, and was followed up by appropriate experiments, during the course of which a chance observation served to trigger off a planned investigation, which then led to the actual discovery. Such a train of events often underlies what is said to be a chance discovery.
I prepared lysergic acid diethylamide for the first time in 1938 as part of a systematic chemical and pharmacological investigation of partially synthetic amides of Iysergic acid in the Sandoz pharmaceutical-chemical research laboratories in Basle, headed at that time by Professor Arthur Stoll. Lysergic acid is the characteristic nucleus of the alkaloids of ergot and can be obtained by alkaline hydrolysis of these alkaloids. Using a newly developed procedure, one had proved it possible to combine Iysergic acid with amines in peptide linkage. In this way, the specific oxytocic principle of ergot, namely Ergometrine, known in this country as ergonovine, was produced. This was the first partial synthesis of a natural ergot alkaloid, and by modifying the alkanolamine side chain of Ergometrine a new synthetic derivative, which we named Methergine, was obtained. In its pharmacological properties Methergine proved to be superior to the natural alkaloid, and today it is used throughout the world in obstetrics for the arrest of hemorrhage. Although interest centered mainly on oxytocic and hemostatic activity in these investigations, the new method of synthesis was also employed to prepare amides of Iysergic acid, which, on the basis of their chemical structure, might be expected to possess different pharmacological properties. Thus among other compounds, I synthesized the diethylamide of Iysergic acid with the intention of obtaining an analeptic. This compound might have been expected to possess analeptic properties because of its structural relationship with the well-known circulatory stimulant nikethamide.
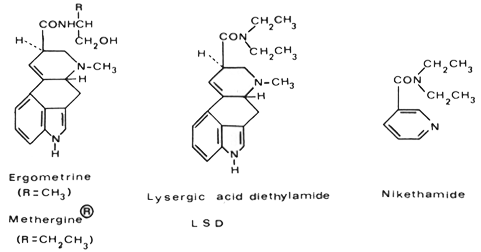
A number of pharmacological experiments were carried out by Professor Ernst Rothlin with this new compound, which was given the laboratory code name LSD-25 because it was the twenty-fifth compound of the Iysergic acid amide series. These experiments revealed a fairly marked uterotonic action, not unexpected in view of the close chemical relationship between LSD and the oxytocic drugs Ergometrine and Methergine. In addition, marked excitation was observed in some of the animals. Work on LSD then fell into abeyance for a number of years.
Because I had the feeling that it would be worth while to carry out more profound studies with this compound, I prepared a fresh quantity of LSD in 1943. In the course of this work an accidental observation led me to carry out a planned self-experiment with this compound. The following is an extract of my report on these experiments, dated April 22, 1943, and addressed to the Head of the Pharmaceutical Department, Professor Stoll.
Last Friday, April 16, 1943, I was forced to stop my work in the laboratory in the middle of the afternoon and to go home, as I was seized by a peculiar restlessness associated with a sensation of mild dizziness. On arriving home, I lay down and sank into a kind of drunkenness which was not unpleasant and which was characterized by extreme activity of imagination. As I lay in a dazed condition with my eyes closed (I experienced daylight as disagreeably bright) there surged upon me an uninterrupted stream of fantastic images of extraordinary plasticity and vividness and accompanied by an intense, kaleidoscope-like play of colors. This condition gradually passed off after about two hours.
The nature and the course of this extraordinary disturbance raised my suspicions that some exogenic intoxication may have been involved and that the Iysergic acid diethylamide with which I had been working that afternoon could have been responsible. I had separated the two isomeric forms that are formed by this synthesis, namely Iysergic diethylamide and isolysergic acid diethylamide and prepared the crystalline water soluble salt of Iysergic acid diethylamide with tartaric acid. However, I could not imagine how this compound could have accidentally found its way into my body in a sufficient quantity to produce such phenomena. Moreover, the nature of the symptoms did not tally with those previously associated with ergot poisoning. In order to get to the root of the matter, I decided to conduct some experiments on myself with the substance in question. I started with the lowest dose that might be expected to have any effect, i.e., 0.25 mg LSD. The notes in my laboratory journal read as follows:
April 19, 1943: Preparation of an 0.5% aqueous solution of d-lysergic acid diethylamide tartrate.
4:20 P.M.: 0.5 cc (0.25 mg LSD) ingested orally. The solution is tasteless.
4:50 P.M.: no trace of any effect.
5:00 P.M.: slight dizziness, unrest, difficulty in concentration, visual disturbances, marked desire to laugh...
At this point the laboratory notes are discontinued: The last words were written only with great difficulty. I asked my laboratory assistant to accompany me home as I believed that I should have a repetition of the disturbance of the previous Friday. While we were cycling home, however, it became clear that the symptoms were much stronger than the first time. I had great difficulty in speaking coherently, my field of vision swayed before me, and objects appeared distorted like images in curved mirrors. I had the impression of being unable to move from the spot, although my assistant told me afterwards that we had cycled at a good pace.... Once I was at home the physician was called.
By the time the doctor arrived, the peak of the crisis had already passed. As far as I remember, the following were the most outstanding symptoms: vertigo, visual disturbances; the faces of those around me appeared as grotesque, colored masks; marked motoric unrest, alternating with paralysis; an intermittent heavy feeling in the head, limbs and the entire body, as if they were filled with lead; dry, constricted sensation in the throat; feeling of choking; clear recognition of my condition, in which state I sometimes observed, in the manner of an independent, neutral observer, that I shouted half insanely or babbled incoherent words. Occasionally I felt as if I were out of my body.
The doctor found a rather weak pulse but an otherwise normal circulation.... Six hours after ingestion of the LSD my condition had already improved considerably. Only the visual disturbances were still pronounced. Everything seemed to sway and the proportions were distorted like the reflections in the surface of moving water. Moreover, all objects appeared in unpleasant, constantly changing colors, the predominant shades being sickly green and blue. When I closed my eyes, an unending series of colorful, very realistic and fantastic images surged in upon me. A remarkable feature was the manner in which all acoustic perceptions (e.g., the noise of a passing car) were transformed into optical effects, every sound evoking a corresponding colored hallucination constantly changing in shape and color like pictures in a kaleidoscope. At about one o'clock I fell asleep and awoke next morning feeling perfectly well.
This was the first planned experiment with LSD and a rather dramatic one. Subsequent experiments on volunteer colleagues of the Sandoz research laboratories confirmed the extraordinary activity of LSD on the human psyche. These showed that the effective oral dose of LSD in human beings is 0.03 mg to 0.05 mg. In spite of my caution, I had chosen for my first experiment five times the average effective dose. LSD is by far the most active and most specific hallucinogen. It is about 5,000 to 10,000 times more active than mescaline, which produces qualitatively nearly the same symptoms. The extremely high potency of LSD is not just a curiosity; it is in many respects of the greatest scientific interest. For example, it lent support to the hypothesis that certain mental illnesses that were supposed until then to be of purely psychic nature had a biochemical cause because it now seemed feasible that undetectable traces of a psychoactive substance produced by the body itself might produce psychic disturbances.
LSD was unique with regard to its extremely high hallucinogenic potency. But it was not new with regard to the quality of its hallucinogenic property. As already mentioned, it produces qualitatively the same psychic effects as mescaline, a hallucinogen known long before LSD, mescaline being the active principle of one of the ancient magic plants of Mexico. Hallucinogenic drugs were of great importance in the old Indian cultures of Central America. The Spanish chroniclers and naturalists who came to the country soon after the conquest of Mexico by Cortes mentioned in their writings a great number of plants with psychic effects. These plants were unknown in the Old World and were used by the Indians both in their medical practices and in their religious ceremonies. The cultic use and divine worship paid to many of these drugs met with the disapproval of the Christian missionaries, who attempted by any means possible to liberate the Indians from this "devilry." They were only partially successful, however, in this respect. The native population secretly continued to use the drugs, which they considered to be holy even after they had been converted to Christianity.
Three main magic plants were used by the Aztecs and neighboring tribes in their religious ceremonies and in their medical practices, which were strongly influenced by magical concepts; these drugs are still used today for the same purpose by the witch doctors in remote districts of Mexico. They are:
1/ peyotl, a cactus species,
2/ teonanacatl, certain foliate mushrooms, and
3/ ololiuhqui, the seeds of certain bindweeds.
The first magic plant to be studied scientifically was peyotl, also named peyote; this was under investigation as early as at the turn of the century. The history of peyotl, its ancient cultic use by the Indians of Central America and its present use, as well as the psychic effects on human beings, were first described by Louis Lewin, the brilliant pharmacologist and toxicologist, in his classic monograph entitled Phantasticka.
Lewin established the basis for psychotomimetic research by this standard publication in which the psychotomimetics or hallucinogens, named by him Phantastika, were characterized and grouped for the first time in an independent group within the psychopharmaceutical drugs. The peyotl cactus was named by the botanist Hennings Anhalonium lewinii in honor of Lewin. Louis Lewin and Arthur Heffter, who isolated the alkaloid mescaline in 1896 from the cactus, deserve a place of honor in the history of psychopharmacological psychotomimetic research. In 1919, Ernst Späth in Vienna succeeded in elucidating the chemical structure of mescaline and in synthesizing this alkaloid.
Mescaline made it possible for the first time to investigate the phenomenon of hallucinogenic effects from a scientific, pharmacological, and clinical aspect using a pure chemical compound. The results of this first period of psychopharmacological investigations in the nineteen twenties were published in the classical monograph Der Meskalinrausch by K. Beringer. However, in the years to follow, interest in hallucinogenic research faded. Not until the nineteen forties with the discovery of LSD did this line of research receive a new impetus leading to an upsurge of interest that has lasted until the present time.
Our preoccupation with LSD was the reason why the second magic drug of Mexico, teonanacatl, which means "sacred mushroom," was submitted to our laboratory for a chemical analysis. The use and worship of teonanacatl by the Indians of Central America must be very ancient. In Guatemala, "mushroom stones" have been found the oldest specimens of which are over three thousand years old.

Fig. 1. Mushroom Stone |

Fig. 2. Psilocybe mexicana Heim |
Although the mushroom cult is very old, our knowledge of it is very recent. For some centuries the reports in the old chronicles were given surprisingly little attention, probably because they were regarded as extravagances of a superstitious age. It was not until 1936 to 1938 that American investigators, i.e., Weitlander, Reko, Johnson and Schultes, ascertained that mushrooms were still being eaten for magical purposes by the natives in certain remote districts of southern Mexico. Systematic studies of the mushroom cult in its present form were later made by the amateur investigators R. Gordon Wasson and his wife. In the summer of 1955, Wasson was able for the first time to take active part in a secret nocturnal ceremony in Huautla de Jimenez Province of Oaxaca, and was probably the first white man to ingest the holy mushrooms. On a later expedition in 1956, Wasson was accompanied by the mycologist Roger Heim, director of the Laboratoire de Cryptogamie du Museum National d'Histoire Naturelle in Paris. Heim succeeded in identifying the sacred mushrooms and classifying them botanically. They belong to the family of Strophariaceae, principally to the genus Psilocybe, though one species belongs to the genus Stropharia and another to the genus Conocybe. Artificial cultivation in the laboratory provided a good yield of especially one of these sacred mushrooms, namely, Psilocybe mexicana Heim.
After unsuccessful attempts in Paris to isolate the active principles, Professor Heim sent the mushrooms to the Sandoz Research Laboratories in Basle, believing that our experience with LSD would enable us to solve this problem. In a sense, therefore, LSD brought the sacred mushrooms to our laboratory.
In the first phase of our isolation studies, we tried to evaluate the extracts by testing them on animals, observing pupillary reaction and piloerection in mice and general behavior in dogs. But the results were not clear-cut and led to discrepancies in the evaluation of the various extract fractions. After most of the very rare and valuable mushroom material had been used for animal testing without definite results, there was some doubt whether the mushrooms cultivated and dried in Paris were still active at all. In order to settle this fundamental point, I decided to test them on myself. I ate 32 dried specimens of Psilocybe mexicana weighing 2.4 g, corresponding to a medium dose by Indian standards. I shall describe my experience by reading the English translation of my original published report of that experiment:
Thirty minutes after my taking the mushrooms, the exterior world began to undergo a strange transformation. Everything assumed a Mexican character. As I was perfectly well aware that my knowledge of the Mexican origin of the mushroom would lead me to imagine only Mexican scenery, I tried deliberately to look on my environment as I knew it normally. But all voluntary efforts to look at things in their customary forms and colors Proved ineffective. Whether my eyes were closed or open, I saw only Mexican motifs and colors. When the doctor supervising the experiment bent over me to check my blood pressure, he was transformed into an Aztec priest and I would not have been astonished if he had drawn an obsidian knife. In spite of the seriousness of the situation, it amused me to see how the Germanic face of my colleague had acquired a purely Indian expression. At the peak of the intoxication, about 1 1/2 hours after ingestion of the mushrooms, the rush of interior pictures, mostly abstract motifs rapidly changing in shape and color, reached such an alarming degree that I feared that I would be torn into this whirlpool of form and color and would dissolve. After about six hours the dream came to an end. Subjectively, I had no idea how long this condition had lasted. I felt my return to everyday reality to be a happy return from a strange, fantastic but quite real world to an old and familiar home.
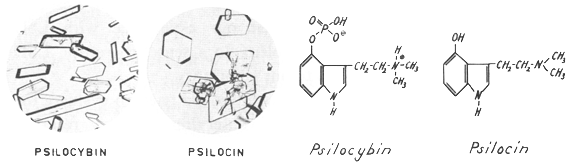 |
| Fig. 3. Crystals of psilocybin and psilocin, and formulas. |
This personal study showed that the negative results of the tests in animals were due not to the mushroom material but to the animals used and that human beings provide a more sensitive index of substances with psychic effects than animals. With the aid of this reliable test in human beings, it was then possible to extract the active principles from the mushroom and to purify and crystallize them. The main active component was named psilocybin, and an accompanying alkaloid, usually present only in small amounts, named psilocin.
The elucidation of their structures showed that these were a novel type of indole derivatives. Psilocybin is the first and only hitherto known natural indole compound that contains a phosphoric acid radical. Furthermore, psilocin and psilocybin were the first indole alkaloids with a free or phosphorylated hydroxyl group at the position 4 of the indole ring system, all the other numerous indole alkaloids bearing hydroxyl groups at the positions 5, 6 or 7.
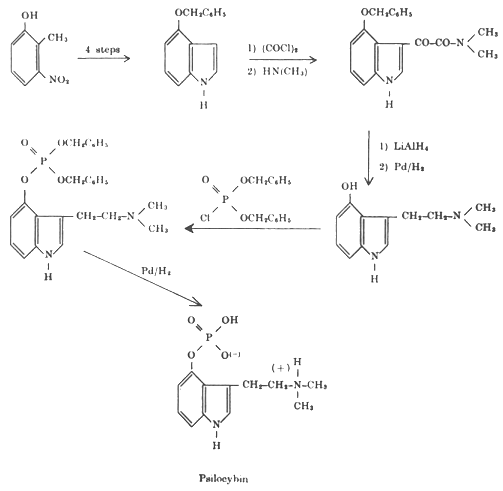 |
The final proof of the correctness of the proposed structures was provided by the total synthesis of psilocin and psilocybin. The synthetic production of psilocybin and psilocin is now more economical than obtaining them from the mushrooms. My colleagues who participated in these investigations were: Dr. Arthur Brack, Dr. Albert Frey, Dr. Hans Kobel, Dr. Hans Ott, Dr. Theodor Petrzilka and Dr. Franz Troxler.
A review of the historical, ethnological, botanical and chemical aspects of the hallucinogenic Mexican mushrooms is presented in the beautiful volume, Les champignons hallucinogènes, by Roger Heim and R. Gordon Wasson, edited by the Museum National d'Histoire Naturelle in Paris. An average human oral dose of psilocybin is 6 mg to 10 mg. Psilocin possesses similar activity. This means that psilocybin and psilocin are about 100 times more active than mescaline and about 100 times less active than LSD. But there is no significant difference between the two compounds in quality of hallucinogenic activity. The development of cross-tolerance between LSD and psilocybin lends support to the view that these two drugs cause psychic disturbances by acting on some common mechanism, or on mechanisms acting through a common final pathway.
When I was in Mexico on an expedition with my friend Gordon Wasson in 1963, in search of a hallucinogenic plant, we also visited the famous curandera Maria Sabina in Huautla de Jimenez. We were invited to attend a nocturnal mushroom ceremony in her hut, but as it was late in the year and no more mushrooms were available, I supplied her with pills containing synthetic psilocybin. She took a rather strong dose corresponding to the number of mushrooms she usually ingests. It was a gala performance assisted by a number of people of Maria Sabina's clan. At dawn when we left the hut, our Mazateca interpreter told us that Maria Sabina had said there was no difference between the pills and the mushrooms. This was a final proof that our synthetic psilocybin was identical in every respect with the natural product.
That was the story of the second magic Mexican drug of teonanacatl. But there was still the riddle of ololiuqui, the third magic Mexican drug. Ololiuqui is the Aztec name for the seeds of certain convolvulaceous plants that since prehispanic times have been used by the Aztecs and related tribes in their religious ceremonies and magic medicinal practices in the same way as the sacred mushrooms and the cactus peyotl. Ololiuqui is still used in our day by such tribes as the Zapotecs, Chinantecs, Mazatecs, and Mixtecs, who live in the remote mountains of southern Mexico in comparative isolation, little influenced by Christianity. An excellent review of the historical, ethnological and botanical aspects of the ololiuqui question was given in 1941 by R. Evans Schultes of the Botanical Museum at Harvard, in his monograph, A Contribution to our Knowledge of Rivea corymbosa. The Narcotic Ololiuqui of the Aztecs.
 |
The first description and illustration of ololiuqui was published by Francisco Hernandez, a Spanish physician, who between 1570 and 1575 carried out extensive research on the flora and fauna of Mexico for Philip II. In his famous Rerum Medicarum Novae Hispaniae Thesaurus, seu Plantarium, Animalium, Mineralium Mexicanorum Historia, which appeared in 1651 in Rome, Hernandez described ololiuqui under the heading "De Oliliuhqui, seu planta orbicularium foliorum." An extract of the translation of the 1651 Latin version reads as follows:
Oliliuhqui, which some call coaxihuitl, or snake plant, is a twining herb with thin, green cordate leaves, slender, green terete stems and long white flowers. The seed is round and very like coriander.... Formerly, when the priests wished to commune with their gods and to receive a message from them, they ate this plant to induce a delirium. A thousand visions and satanic hallucinations appeared to them....
 |
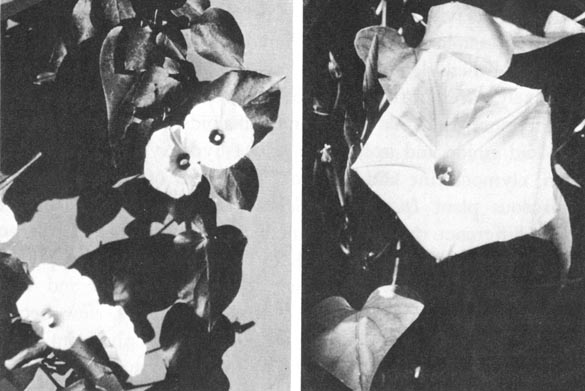
| Fig. 5. Seeds of Ipomoea tricolor and Rivea corymbosa |
The only report of chemical studies on the seeds of Rivea corymbosa mentioned in Schultes' review on ololiuqui is that of the pharmacologist Santesson in Stockholm in 1937. He was, however, unsuccessful in isolating definite crystalline compounds. Alcoholic extracts produced a kind of narcosis or partial narcosis in frogs and mice.
In 1955, the Canadian psychiatrist Osmond conducted a series of experiments on himself. After taking 60 to 100 Rivea seeds he passed into a state of apathy and listlessness accompanied by increased visual sensitivity. After about four hours, there followed a period in which he had a relaxed feeling of well-being that lasted for a rather longer time. In contrast to these results, KinrossWright in 1958 published experiments performed on eight male volunteers who had taken doses of up to 125 seeds without any ascertainable effect.
After the chemical investigations of the sacred Mexican mushrooms had been successfully brought to a close, I decided to tackle the problem of the third Mexican magic drug, ololiuqui. Through the help of R. G. Wasson, I was able to obtain authentic ololiuqui from a Zapotec Indian near Oaxaca in southern Mexico. One sample consisted of brown seeds, which proved on botanical classification to stem from Rivea corymbosa. The black seeds of the second sample were identical with those of Ipomoea violacea L. (syn. Ipomoea tricolor CA V.). These black seeds, called "badoh negro," arc used especially in the region of the Zapotecs, in conjunction with, or instead of "Badoh," the brown seeds of Rivea corymbosa.
The chemical analysis of the ololiuqui seeds gave a quite surprising result. The psychotomimetic principles that we isolated proved to be Iysergic acid derivatives, Iysergic acid amides and other ergot alkaloids. Thus in this strange Mexican drug we met with old friends, since Iysergic acid derivatives and ergot alkaloids had been favorite subjects of research in our laboratory since the time I had first synthesized LSD in the nineteen-thirties.
 |
| Fig. 6. Flowering plants of Rivea corymbosa and Ipomoea tricolor. |
The main constituents of ololiuqui, i.e., the seeds of Rivea corymbosa, are lysergic acid amide and lysergic acid 1-hydroxyethylamide. The active constituents of ololiuqui are very closely related to LSD. The only difference between the main constituent of ololiuqui, Iysergic acid amide, and LSD is that the two hydrogen atoms of the amide are replaced by two ethyl radicals. It is a very significant one with regard to the psychotomimetic activity. The ololiuqui principle, Iysergic acid amide, is much less active than Iysergic acid diethylamide (LSD), and provokes psychic symptoms qualitatively different from those of LSD, as will he shown later.
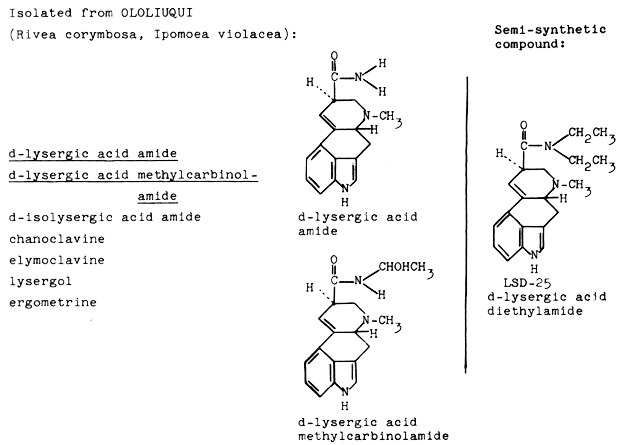 |
| Fig. 7. Structural relation between active principles of ololiuqui and LSD-25. |
Furthermore, the following minor alkaloids were isolated: isolysergic acid amide and isolysergic acid 1-hydroxyethylamide, chanoclavine, elymoclavine and lysergol. The seeds of the related convolvulaceous plant Ipomoea violacea yielded the same alkaloids with the difference that ergometrine was present instead of lysergol. The occurrence of ergot alkaloids in higher plants, in the phanerogamic family Convolvulaceae was quite unexpected and is of particular interest from the phytochemical point of view because Iysergic acid alkaloids had hitherto been isolated only from genera of lower fungi: Claviceps, Penicillium and Rhizopus. Lysergic acid amide, the main component of ololiuqui, had been tested pharmacologically and clinically under the experimental drug designation LA-111 during the course of our investigations on LSD and related compounds long before it was known to be a natural component of a magic Mexican drug. Self-experiment and comparative systematic clinical investigations with Iysergic acid amide (laboratory code name: LA-111) revealed psychotomimetic effects significantly different from those of Iysergic acid diethylamide (LSD-25). The symptoms after oral ingestion of 1 mg to 2 mg of LA-111 were: indifference, decrease of psychomotor activity, tiredness, feeling of sinking into nothingness, and desire to sleep. Isolysergic amide produces similar symptoms. After taking 2.0 mg of isolysergic amide orally, I experienced tiredness, apathy, a feeling of mental emptiness and of the unreality and complete meaninglessness of the outside world. These comparative experiments showed that the psychotomimetic constituents of ololiuqui are 20 to 40 times less active than LSD and that the general picture of activity is characterized by a pronounced depressive and narcotic component.
I now come to the last section of my chapter; here I shall discuss very briefly some common features in the chemical structure of the hallucinogens I have discussed. The comparison of these structures reveals an interesting relationship with the structures of important neurohumoral substances. This is certainly no mere coincidence, but of major importance.
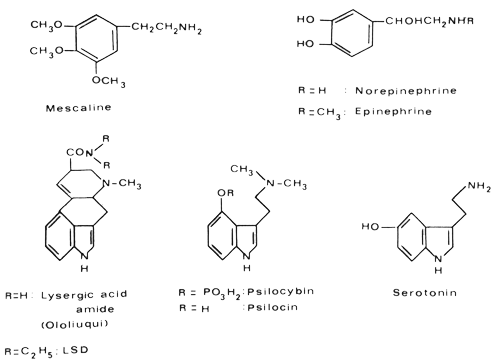 |
Mescaline, being a phenylethylamine derivative, is structurally related to the neurohumoral transmitters norepinephrine and epinephritic. LSD, and the constituents of ololiuqui as well as the active principles of the hallucinogenic mushrooms psilocybin and psilocin, are indoles, more precisely tryptamine derivates, like the neurohumoral factor serotonin. Because of this structural relationship between the hallucinogens and norepinephrine and serotonin, it is probable that the psychotomimetic activity is due to an interaction between these substances in the metabolism of the central nervous system. Investigation of the relationships between endogenous neurohumoral factors and hallucinogens is a rewarding facet of psychopharmacological research.
As I am a chemist, I have mainly discussed the chemical, phytochemical and historical aspects of the discovery of LSD and the investigation of naturally occurring hallucinogens. Needless to say, this audience attaches primary importance to the pharmacological and clinical effects, which make LSD and the other specific hallucinogens a valuable tool in experimental psychiatry and a valuable drug aid in psychoanalysis and psychotherapy. Another aspect of the hallucinogens and especially of LSD with enormous social impact is of course the paramedical use and the misuse of these substances. But this very complex problem would provide material for a special lecture, or indeed for a series of such lectures.
The aim of my chapter is to describe an unusual cycle of chemical research, full of coincidences, a kind of magic circle, which started with the synthesis of various Iysergic acid amides and the discovery of the extraordinary psychotomimetic potency of Iysergic acid diethylamide (LSD), which led to the investigation of the sacred Mexican mushrooms, the isolation of psilocybin, and ended with ololiuqui, where lysergic acid amides were again encountered, thus closing the magic circle.
REFERENCES
- Lewin, L.: Phantastika, Berlin, Georg Stilke, 1924. Second enlarged edition published in 1927. The first English edition appeared in 1931. A new impression 1964: Phantastica, Narcotic and Stimulating Drugs, Their Use and Abuse, London, Routledge and Kegan Paul, 1964.
- Heffter, A.: Ber 29:216, 1896.
- Späth E.: Monatsh 40:129, 1919.
- Beringer, K.: Der Meskalinrausch, Berlin, Springer, 1927.
- Hofmann, A., et al.: Helv Chim Acta 42:1557, 1959.
- Heim, R., and Wasson, R. G.: Les Champignons hallucinogènes du Mexique, ed. du Muséum national d'Histoire Naturelle, Paris, 1958.
- Schultes, R. E.: A Contribution to Our Knowledge of Rivea Corymbosa: The Narcotic Ololiuqui of the Aztecs, Botanical Museum, Harvard Univ, Cambridge (Mass), 1941.
- Osmond, H.: J Ment Sci 101:526, 1955.
- Kinross-Wright, V. J.: In Bradley, P. B., Deniker, P., and RaduocoThomas C., eds.: Neuro-Psychopharmacology, Amsterdam, Elsevier, 1959, p. 453.
- Hofmann, A.: Planta Med 9:354, 1961.
- Hofmann, A.: Botan Museum Leaflets, Harvard Univ 20:194, 1963.
|
|
Last Updated on Monday, 20 December 2010 21:51 |
|









