| Articles - Addiction |
Drug Abuse
Addiction: brain mechanisms and their treatment implications
David J Nutt
University of Bristol
Psychopharmacology Unit
School of Medical Sciences
University Walk, Bristol BS81TD, UK
(D J Nutt FRCPsych)
The Lancet 1996; 347: 31-36
Drug addiction, or as it is also called, drug dependence, is a serious health problem; in addition to the huge direct health costs (psychiatric and physical), there are massive costs in terms of crime, loss of earnings and productivity, and social damage. The drugs of primary concern are the opioids, stimulants (amphetamines, cocaine), and alcohol, although nicotine addiction (smoking) is also an important health issue. Reducing the extent of drug dependence is one of the major goals of medicine.
The processes of addiction involve alterations in brain function because misused drugs are neuroactive substances that alter brain transmitter function. Then is an impressive and rapidly growing research base that is giving important insights into the neurochemical and molecular actions of drugs of misuse--the processes that are likely to determine such misuse in human beings. Exciting new developments in neuroimaging with both PET (positron emission tomography) and SPELT (single photon emission computed tomography) provide, for the first time, the possibility of testing in human beings theories of drug addiction derived from preclinical studies. Key concepts of addiction are shown in the panel.
Panel: Key concepts
Drug dependence Because addiction is an imprecise and potentially pejorative term. the WHO recommended In 1969 that It should be replaced by the term drug dependence. Dependence is a continuous variable; for any Individual Its extent is determined by a range of factors such as amount and frequency of drug use, development of tolerance and withdrawal, inability to abstain, and degree of physical, personal, and social damage. The dependence spectrum thus ranges from simple physical dependence, as for example in some long-term therapeutic-dose benzodiezepine users, to the complete disintegration of personal and social functioning found in end-stage alcoholics and 'hard drug' users. Physical dependence is caused by alterations in brain function that lead to the experiences of withdrawal. Psychological dependence describes repeated drug seeking and taking in the absence of withdrawal. Both can occur independently and contribute differing amounts to dependence on different drugs.
Tolerance This is the state in which drug actions diminish on repeated administration. Tolerance means that the addict needs more drug per dose; this increase in cost drives criminal activities. Tolerance often develops at a different rate for different actions of the drug. The respiratory depression caused by opioids reduces faster than the euphoric actions; this explains why addicts can use doses of heroin that would be lethal to non-addicts (several grams a day). A person becomes tolerant to the euphoric actions of cocaine faster than to its cardlostimulant actions, so on binge use, cardlotoxic concentrations are frequently reached.
Withdrawal Withdrawal Is signified by signs and symptoms that occur when a drug Is stopped or an antagonist (eg, naloxone for opiolds) Is given. Both physiological and psychological (conditioning) processes contribute. Because withdrawal Is almost invariably unpleasant (eg, morning shakes with alcohol) I Is a common reason for reuse of a drug. Moreover, withdrawal may also cause secondary problems. Examples are excitotoxic brain damage in the case of alcohol, and depression and anxiety in the case of cocaine. Withdrawal from some drugs (eg. methadone) may be long lived, and can be associated with continued craving.
Sensitisation This Is the opposite of increase In some actions of a drug on repeated administration--and tends to be seen with the stimulating actions of drug states. One example Is the increased locomotor activating effects of cocaine and amphetamine; a clinical corollary of this may be the psychotic state seen during stimulant binges. Both probably reflect dopamine receptor supersensitivity. Sensitisation to the excitatory changes found in withdrawal also occurs and this explains the long-established clinical observations that alcohol withdrawal progressively worsens. Sensitisation may also be the process of reinstatement, in which as an addiction 'career' progresses, relapses escalate much more rapidly to a state of decompensatlon.
Craving Craving, the desire to get (more of) the drug, Is difficult to define because It has several subcomponents. which differ between drug and between Individuals. For instance, with stimulants and alcohol, the first dose of drug can lead to a euphoric priming that drives repeated consumption: In many opioid addicts and alcoholics, craving is associated with withdrawal symptoms that seem to be conditioned to significant aspects of previous drug use-eg, needles and syringes or bars. Craving often leads to the addict's behaviour becoming highly focused on getting the drug with a narrowing of the normal behavioural repertoire. For example, an alcoholic will spend more and more time thinking about and engaging in drinking, and this leads to a progressive reduction In participation in work and family activities.
Euphoria Known by mart' synonyms (eg, rush, high, buzz) euphoria Is the state of pleasure produced by a drug. This state is closely linked to the reinforcing effects of the drug (is, how likely It is to lead to continued use). Euphoria is thought to relate to endogenous dopamine and or endogenous opiod release and is determined by both pharmacodynarnic and pharmecokinetic factors. Euphoria is not the sole reason for the use of drugs. Many addicts start drug use to deal with psychiatric difficulties, especially anxiety, which sedatives, opioids, and even stimulants can reduce.
Maintenance therapy In this therapeutic approach, the need for Illicit drug use is removed because the addict Is prescribed a drug whose actions substitute for the drug of misuse. Maintenance therapy Is an important component of harm reduction programmes, which seek to reduce the personal and social cost of drug addiction when abstinence Is not an option. The best example Is with oplold addiction, in which methadone reduces HIV infection and crime as well as engages the addict in treatment. Methadone has the disadvantage of the need for daily prescription and a high diversion potential into street (black market) use. Longer acting alternatives under investigation are buprenorphine and LRAM (bawetylmethadol); these can be given every 2-3 days, have lower street value, and are safer In overdose. Mcotine gums and patches are a safer route for nicotine self-administration than Is smoking. It is likely that maintenance therapy can be used for most drugs of dependence although clinical trials would be needed. Examples would be use of methylphenidate for cocaine or amphetamine dependence and long-acting benzodiazepines for intravenous temazepam users.
Pharmacological aspects
Drugs of misuse were traditionally classified into groups according to their physiological or psychological actions (eg, stimulants, sedatives). This classification is unsatisfactory because a single drug may have several actions; alcohol often acts as a stimulant in the early (rising) phase of intoxication, but as brain concentrations increase sedation ensues. The molecular sites of action of most drugs of misuse have been well characterised in recent years (see table) so it is preferable to classify drugs according to their pharmacodynamic actions.
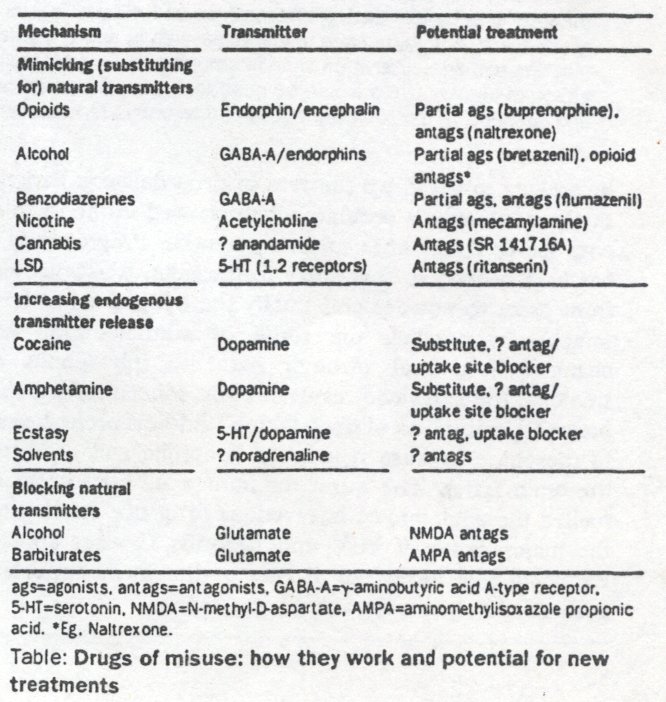
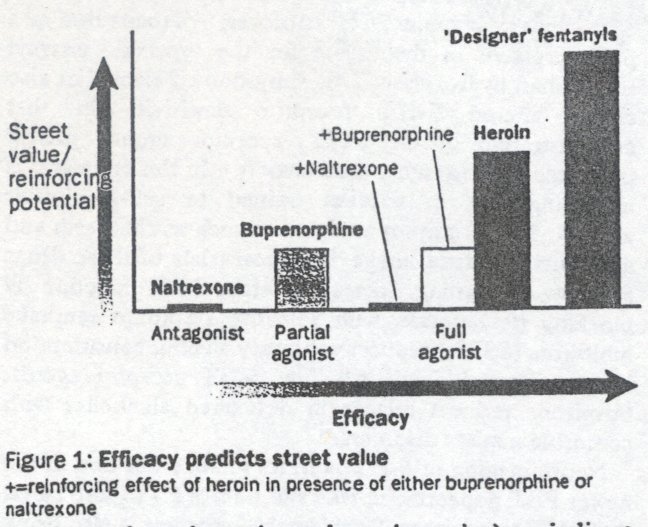
Generally, the more efficacious the drug is at producing its pharmacological effect, the greater is the addiction potential and street value (figure 1). Drugs with lower efficacy are called partial agonists (eg, buprenorphine for opioid receptors) bretazenil for benzodiazepine receptors2). The pharmacological profile of partial agonists is such that they are useful in maintenance treatment since they provide some reinforcement; thus, buprenorphine will keep opioid addicts in treatment. Nonetheless, because partial agonists attenuate the actions of full agonists, buprenorphine should diminish intravenous street heroin use. Moreover, the lower efficacy means that it is much safer in overdose.
Antagonists have zero efficacy (eg, naltrexone for opioid receptors, 3 flumazenil for benzodiazepine receptors4). They are very effective blockers of agonists. Their limitations are that they can precipitate withdrawal in physically dependent addicts and, because they do not provide any reinforcement, there is little incentive for addicts to stay on them. Naltrexone is useful in highly motivated individuals in whom relapse to opioid use could portend the end of their career (eg, doctors and pharmacists). This antagonist is also used in some countries under probation orders when non-compliance with treatment will then lead to prison.
For most drugs of misuse, the molecular sites of action are receptors or transporter sites; many of these have been cloned and sequenced, discoveries which in themselves are important advances for molecular biology. The dopamine transporter was cloned to expedite the understanding of cocaine's action;5 all three of the opioid receptors and the multiple subunits of the γ-aminobutyric acid agonist A-type (GABA A) receptor have also been cloned. Such discoveries help direct research towards a more rational design of treatment, and help develop theories of the brain mechanisms underlying addiction. There is a large research effort being directed towards finding a drug that will bind to the dopamine transporter and prevent the binding, and hence the actions, of cocaine without interfering with dopamine uptake.6
New μ opioid receptor drugs with potential treatment use such as antagonists (clocinnamox)7 or partial agonists (buprenorphine) have been designed. The knowledge that alcohol acts through GABA and excitatory aminoacid receptors is leading to the study of drugs acting at these receptors (eg, acamprosate)8 as treatments.
Pharmacokinetic factors are also important in determining the misuse potential of drugs; in general the faster the drug enters the brain the more reinforcing it is. Many of the developments in drug misuse reflect efforts by addicts to speed up the rate of drug delivery. Perhaps the best example is cocaine; when chewed in the form of coca leaves it has little misuse potential. Progressively it has been refined so that entry to the brain is accelerated, from paste to powder and finally the lipophilic free-base (crack). In parallel, the route of administration has changed from oral through nasal to intravenous or smoking; the latter two result in brain concentrations that peak within minutes of drug taking.9 Addicts prefer heroin to morphine because it is more lipophilic and so enters the brain faster. The quest for immediate reinforcement fuelled the epidemic of intravenous drug use that is now the major cause of HIV and hepatitis C virus spread. Reducing this behaviour is one of the most important goals in addiction treatment programmes.
Brain transmitters involved in addiction
Drugs are used because they produce alterations in brain function that result is positive changes in mood; this can be an elevation in mood from normal (euphoria) or the reduction of a negative dysphoric mood as in withdrawal. These changes are effected by interactions with neurochemical processes, usually by mimicking or increasing the action of endogenous transmitters.
Dopamine
Most drugs that produce elevations of mood or euphoria, including nicotine and alcohol, release dopamine in either the nucleus accumbens or the prefrontal cortex in animals, as demonstrated by brain dialysis.10 Dopamine release can be either direct (for example, the stimulants cocaine and amphetamines release dopamine), or indirect (opioids switch off the firing of GABA neurons that tonically inhibit dopamine cell firing). Several studies have shown that blockade of either D1 or D2 dopamine receptors attenuates the reinforcing actions of both these classes of drugs, which argues for a central mediating role of dopamine receptor activation in the initiation of addiction.11 Of clinical relevance is the suggestion that a genetic polymorphism of the D2 receptor is strongly Baked to drug misuse but this is still controversial.12
Homoeostatic adaptation occurs to the dopamine-increasing actions of drugs, so that when the drug is stopped dopamine release is decreased below normal; this explains the "crash" after stimulant discontinuation 9 and some aspects of nicotine, opioid, and alcohol withdrawal. Drugs that block dopamine reuptake (eg, desiptramine and mazindol, which are used to treat cocaine withdrawal) presumably work by increasing dopamine concentrations.9 Dopamine overactivity probably underlies alcoholic delirium tremens and the need to treat with dopamine-D2-receptor blocking agents such as haloperidol.13 Chronic dysregulation of dopamine function in detoxified alcoholics as revealed by a decreased number of uptake sites in SPECT studies with 123I-βCIT14 may explain the new finding that the low potency neuroleptic tiapride reduces relapse.' ,
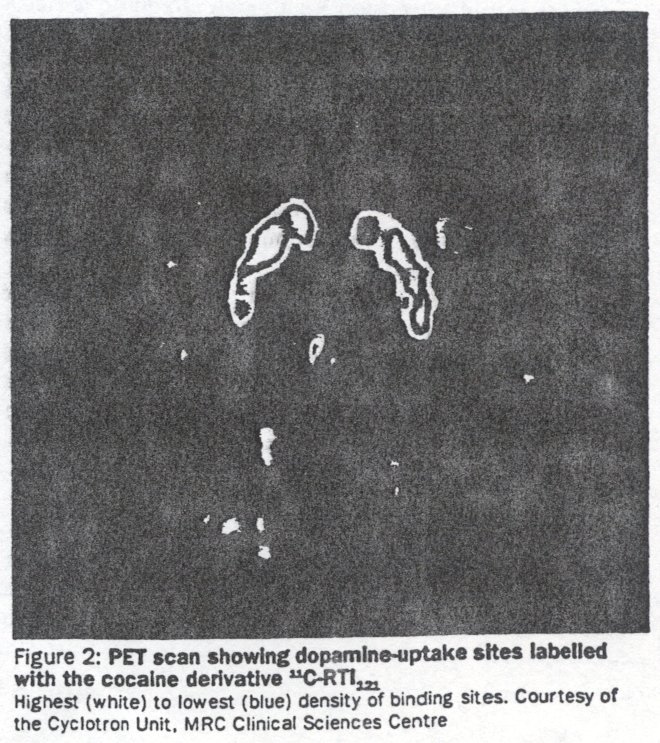
It is now possible to measure both D1 and D2 dopamine receptors and the dopamine uptake site in human beings with neuroimaging techniques (figure 2). Cocaine has been shown with PET to bind predominantly to the dopamine-rich areas of the basal ganglia;16 11C-RTI, an isopropyl derivative of cocaine, is a newer and better marker of these dopamine uptake sites. Now that D2 receptors (with 11C-raclopride) and Dl receptors (with 11C-SCH-23390) can be visualised, alterations in function in addicts could be studied.16 Dopamine metabolism can also be monitored in vivo with 18F-dopa. Similarly, the local metabolic effects of cocaine can be studied with 16F-deoxyglucose uptake. These studies have shown that cocaine globally decreases brain metabolic activity.17 An exciting potential development of PET/SPELT technology is to measure endogenous dopamine release; if cocaine and amphetamine do act by releasing dopamine this should be seen as a displacement of radiolabelled receptor ligand.
Endogenous agonist opioids
The brain makes a complex mixture of peptides that as as endogenous transmitters at opioid receptors-the β-endorphins and encephalins; these are involved in appetite, pain, and response to stress.17 Misused opioids such as heroin act at the same receptors as the natural opioid system. However, because they have much higher efficacy than the endogenous transmitter they "high jack" the natural system by producing a much exaggerated response. Endogenous opioids are thought to be involved in the actions of other misused drugs such as alcohol and stimulants. For example, alcohol may cause dependence because it releases endogenous opioids; this could explain the therapeutic benefit of opioid antagonists such as naltrexone.18
There are three types of opioid receptors (μ, κ and δ) that are distinguished by selective agonists and in some cases antagonists. μ and/or δ receptors mediate the euphoric actions of opioids,19 with δ being possibly more important for alcohol.20 Activation of κ receptors is aversive and could explain some aspects of opioid actions including the dysphoria of withdrawal 21 Many misused opioids have activity at all three receptor types so adaptive changes in each may be important in the process of addiction.
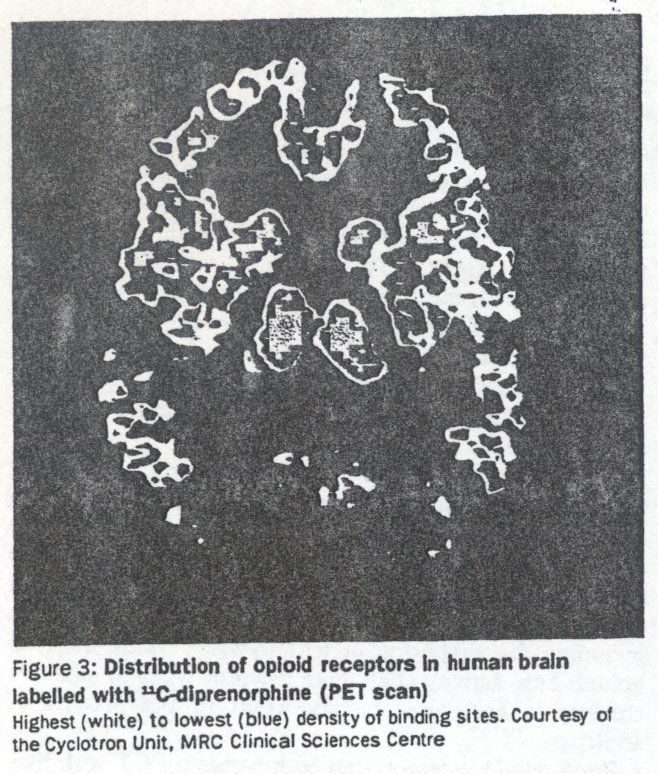
Brain opioid receptors can be imaged in PET with 11C-labelled diprenorphine (non-selective antagonist) (figure 3) or carfentanyl (μ agonist).16 Diprenorphine has been used to show the release of endogenous opioids in some forms of seizures22 and so could potentially be used to reveal whether non-opioid drugs cause their release also. Diprenorphine could also be used to determine the degree of receptor occupation required for optimum therapy with the various maintenance treatments such as methadone and naltrexone. Neuroimaging techniques not only offer the opportunity to test directly theories of drug dependence developed from animal studies in human beings; they can also be used to understand and optimise current treatments and develop new ones. For instance, the proportion of brain opioid receptors occupied during maintenance therapy with methadone could be determined and related to degree of dependence, craving, and treatment outcome. The linking of the clinical effects of partial agonists and their brain binding should lead to the more rational design of new compounds.
Another interesting possibility would be to explore the role of endogenous opioids in craving once reliable methods of inducing this state have been developed. One major future need for opioid receptor neuro-imaging is the development of subtype-selective antagonist ligands to unravel the role of μ, δ and κ receptors in the actions of the various drugs of misuse.
Noradrenaline
The activity of noradrenergic neurons is decreased by opioids, and withdrawal is thought to be due in part to the unopposed expression of compensatory processes. This explains why clonidine 23 or lofecidine, 24 α2-adrenoceptor agonists that inhibit noradrenergic neuronal activity, are effective treatments of opioid withdrawal. A similar hyperadrenergic state accounts for many features of alcohol withdrawal, especially anxiety, tremor, sweating, and hypertension,25 although clonidine is not a recommended treatment for this condition because it does not protect against seizures. Some clinical data suggest that longer-term reduction in noradrenaline activity may predispose alcoholics to relapse and that drugs that selectively reverse this process may have clinical use.26 Complex time-dependent alteration in noradrenaline function has recently been reported in patients withdrawing from stimulants27 which may open new therapeutic avenues.
Recent animal data suggest that noradrenaline/dopamine interactions in the nucleus accumbens and frontal cortex may be important in the actions of stimulant drugs, contributing to features such as sensitisation..26 As yet neuroimaging of brain noradrenaline systems is not possible, although a potential α2-adrenoceptor ligand has been identified.26
Serotonin (5-HT)
5-HT is an amine transmitter secreted by cells whose bodies are found in the raphe nuclei of the brain stem and whose axons arborise and diffusely innervate higher brain structures, as is the case for noradrenaline. 5-HT has many roles in brain function, but in relation to addiction the main ones relate to appetite, impulsivity and craving. Early-onset (type II) alcoholics with a history of violent crime have low brain 5-HT turnover,30 perhaps due to a polymorphism in their gene for the synthetic enzyme tryptophan hydroxylase. This subgroup of alcoholics also shows altered 5-HT receptor sensitivity in that administration of the 5-HT 2 receptor agonist mCPP produced craving rather than anxiety.31 In this context it is intriguing that in rodents trained to self-administer alcohol, 5-HT receptor antagonists such as ritanserin and amperozide reduce intake.32 Clinical trials of these drugs an now continuing. Increasing brain 5-HT function by blocking its reuptake with selective serotonin reuptake inhibitors (SSRIs) reduces voluntary alcohol consumption in heavy social drinkers.33 The 5-HT receptor agonist buspirone reduces relapse in detoxified alcoholics with comorbid anxiety disorders.34
Neuroimaging of 5-HT is in its infancy but one of the newer PET dopamine uptake site tracers RTI 55 also labels the 5-HT transporter. Considerable progress is also being made towards producing an 10F-labelled precursor for turnover studies. 5-HT, receptors have been imaged with 11C-ritauserin,16 although not yet in addicts, and a PET ligand for 5-HT1A receptors ( 11C-WAY 100635) is under development.35
Aminoacid receptors
The major excitatory and inhibitory transmitters in the brain are the closely related aminoacids GABA (inhibitory) and glutamate (excitatory). The GABA receptor complex contains a binding site for the bemodiazepines, which is their sole site of action. Alcohol(s) and the barbiturates also enhance GABA function but in addition block some glutamate receptors.37 This dual action probably explains their added toxicity and dependence liability. Children at bid risk of becoming alcoholics seem to have altered benzodiaepine receptor sensitivity.36
On repeated use of the alcohol(s) and barbiturates, there is a compensatory increase in the number of brain glutamate receptors which contributes to the hyperexcitable state found in withdrawal. Since excessive glutamate activity can be neurotoxic, one suggestion is that repeated withdrawal, as seen nightly in alcoholics, may explain the brain damage in heavy drinkers.37 Brain excitatory , aminoacid receptors are involved in dependence on other drugs; thus, tolerance to opioids can be attenuated by co-treatment with dizocilpine, a blocker of the N-methyl-D-aspartate (NMDA) class of glutamate receptor.38 Because NMDA receptors are very important in processes such as learning and memory, this suggests that drugs of this class could be used to treat some aspects of addiction, especially conditioned responses. Acam-prosate has been suggested to act in this way in alcoholics.
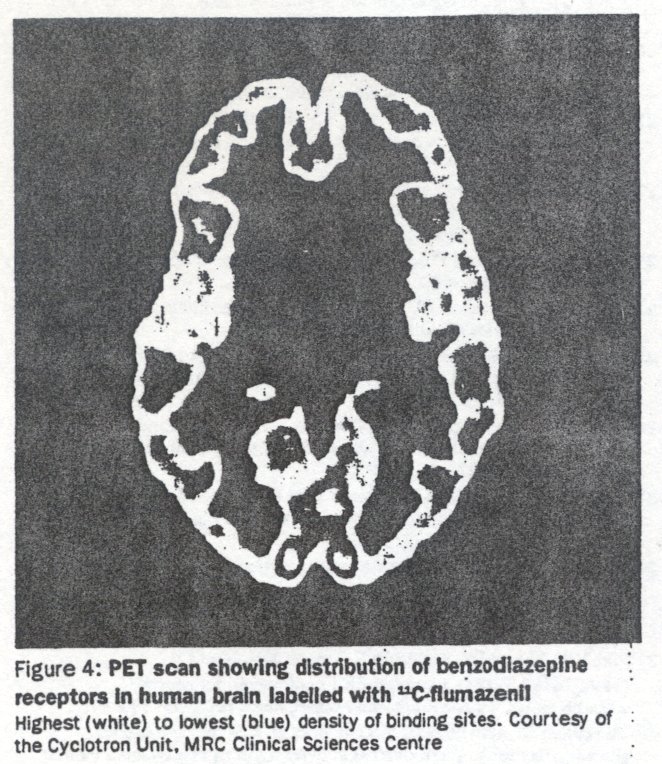
The benzodiazepine site on the GABA-A receptor can be well visualised by PET with 11C-flumazenil39 or SPECT with 123I- iomazenil,40 which are both antagonists. Recent studies have revealed that intoxicating doses of benzodiazepine agonists occupy only about 30% of brain receptors (figure 4) 41 It would be of interest to determine whether this fraction is altered after chronic use, especially high-dose intravenous use as found with many addicts. The brain circuits involved in benzodiazepine withdrawal in animals are parts of the limbic system and thalamus;42 these studies used the deoxyglucose technique which is applicable to PET. ' As yet there are no satisfactory neuroimaging ligands for excitatory receptors, although ketanmine can be used to block NMDA receptor function in patients and could be used in imaging studies of brain metabolism
Other transmitters
There are at least 80 other brain neurotransmitters, some of which are likely to be involved in addiction. One good candidate is CCK (cholecystokinin), which is found in larger amounts in the brain than in the gut. There are two subtypes of brain CCK receptors (A and B), and selective antagonists for each are now available. CCKB-receptor activation seems to be involved in withdrawal from a range of drugs including benzodiazepines, alcohol, and cocaine since antagonists (such as PD 134308) block several aspects of this syndrome. 43 CCK antagonists also moderate tolerance development to opioid analgesia so might have some use in opioid addiction.
Recently, receptors for cannabis have been discovered; one is found predominantly in the brain and the other in peripheral tissues, especially spleen. Both are members of the family of receptors that are coupled to G-proteins which includes the receptors for dopamine, noradrenaline, and many 5-HT receptors. Intriguingly the cannabis receptor in the brain is by far the most abundant of these which suggests an important role in brain function .44 Several possible endogenous transmitters for these receptors have been suggested, with anadamide being the best candidate at present.45 The role of endogenous cannabinoids in addiction can now be tested since a selective antagonist to this receptor has been synthesised. 46
Calcium channels
The regulation of intracellular calcium homoeostasis is critical to all cells and several different calcium channels control the passage of this ion across cell membranes. One of these, the L- type channel, is substantially altered by alcohol and some other misused drugs. Alcohol administration reduces calcium entry through these channels; this results in an adaptive increase in their number so that in withdrawal calcium flux is excessive. Calcium-channel antagonists of the dihydropyridine type (eg, nitrendipine) block some aspects of alcohol withdrawal and when given with the alcohol prevent the increase in channel number.47 The clinical implications of such findings are potentially very important and should be investigated especially since increased calcium flux could also contribute to neuronal death.
Brain circuits of addiction
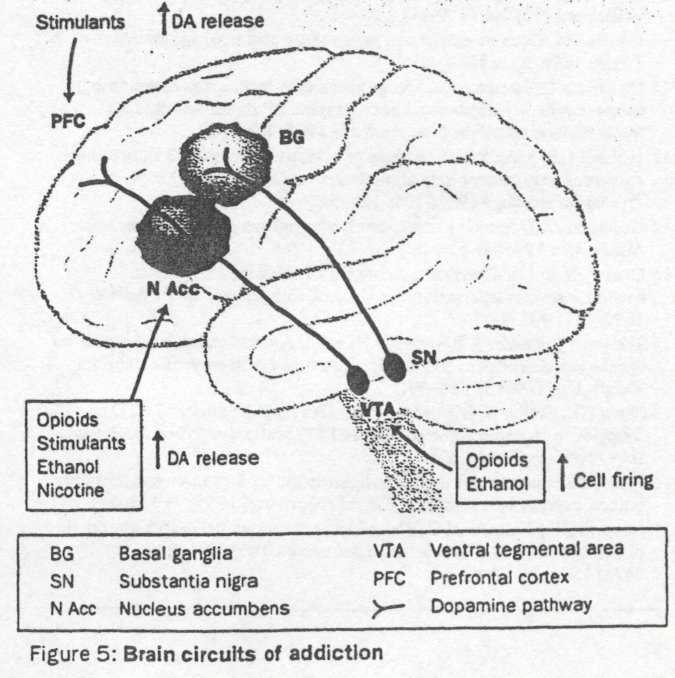
Such brain circuits are beginning to be understood in animals, though there are little supporting data from human beings. The primary circuit seems to be the dopamine pathway that runs from the ventral tegmental area (VTA) through the nucleus accumbens to the prefrontal cortex (figure 5).19 Dopamine release in either the nucleus accumbens, prefrontal cortex, or both is produced by all misused drugs apart from the benzodiazepines.10 Some (eg, cocaine) act on the dopamine terminals, whereas others (eg, opioids) increase cell firing at the level of the cell bodies. Direct injection of drugs into these brain regions is reinforcing since animals will self-administer opioids and cocaine directly into them. Moreover drug withdrawal is associated with reduced dopamine transmission in these regions and aversive drugs (eg, κ agonists) inhibit dopamine release there. 10 ,21 Paradoxically some other aversive experiences such as pain cause dopamine release, and some argue that changes in this transmitter reflect not simply the reinforcing actions of drugs but their salience as a conditioned cue. The feasiblity of measuring dopamine release in human brain by displacement of radioligands has already been mentioned and requires exploration.
Other brain regions important in addiction are the globes pallidus and the amygdala (both of which receive projections from the nucleus accumbens), and the monoaminergic nuclei of locus coeruleus and raphe." Significant changes in transmitter function in these regions have been found with opioids and stimulants in rodents. The pathways have been mapped with markers for altered metabolism (deoxyglucose) and expression of gene products after neuronal activation (eg, cFOS, cJUN). Although many of these brain areas are small in human beings, it is possible to detect alterations in brain metabolism by measuring changes in regional blood flow in some areas (eg, frontal cortex) using PET (15O-water or 18F-dtoxyglucose) or SPELT (99mTc HMPAO). These procedures can also be applied to explore the brain regions that' are activated or shut off during other drugrelated states such as craving and withdrawal. New techniques of image analysis (correlation and spectral analysis) and improved PET camera technology should allow regions such as the nucleus accumbens, and even brainstem structures such as the VTA and the locus coeruleus, to be imaged in future.
Conclusions
Major advances in the science of addiction have been made in the past two decades. We now have a good understanding of the molecular pharmacology of most drugs of. misuse, the only exception being inhaled solvents. The neurobiology of addiction in animals is becoming clearer with the use of new techniques such as drug self-administration. The growth of neuroimaging techniques offers the exciting possibility that the hypotheses developed from the preclinical studies could, and hopefully will, be tested in human beings.
I thank Prof JLewis and Dr A Malizia for their help with this article.
References
1 Lewis Jw Buprenorphine. Drug Alcohol Dep 1985;14: 363-72.
2 Busto U, Kaplan H, Zawertallo 1, Seller EM. Pharmsocologic effects and abuse liability of bretazenil, diazepam, and alprazolam in humans. Clin Pharmocol Ther 1994, 55: 451-63.
3 Judson BA, Goldstein A. Naltrexone treatment of heroin addiction: one-year follow-up. Drug Alcohol Dep 1984;13: 357-65.
4 Hunkeler W, Mohler H, Pieri, L et.a1. Selective antagonists of benzodiazepines. Nature 1981; 290: 515-16.
5 Shimada S, Kitayama S, Lin C, a al. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science 1992; 2S6 576-78.
6 Rothman RB, Mele A, Reid AA, a al. GBR12909 antagonizes the ability of cocaine to elevate extracellular levels of dopamine. Pharmacol Biochem Behav 1991; 40: 387-97.
7 Aceto MD, Bowman ER, May EL, a al. Very long-acting narcotic antagonists: the 14B-p-substituted cinnomoylammomorphinones and their partial mu agonist codeinone relatives. Arzneim-Forsch/Drug res 1989; 39: 570-75.
8 Littleton J. Acamprosate is alcohol dependence: how does it work" Addiction 1995; 90:1179-88.
9 Gawin FH. Cocaine addiction: psychology and neurophysiology. Science 1991; 253:1580-86.
10 Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentration in the mesolimbic system of freely moving rats. Proc Natl Acad Sci 1988; SS% $274.
11 Hubner CB, Moreton JE. Effects of selective D1 and D2 dopamine antagonists on cocaine self-administration in the rat Psychopharmacology 1991; 105 151-56.
12 Goldman D. Dopamine transporter, alcoholism and other diseases. Nature Med 1995; l: 624-25.
13 Glue P, Nutt DJ. Overexcitement and disinhibition: dynamic neurotransmitter interactions in alcohol, withdrawal. Br J Psychiatry 1990;157 491-99.
14 Tlihonen J, Kuikka J, Bergstrom K. et al. Altered striatal dopamine reuptake site densities in habitually violent and non-violent akoholics. Native Mod 1995;1: 654-57.
15 Shaw GK Waller S, Malumdar SK, Alberta JL. Latham CH, Dunn G. Tiapride in the prevention of relapse in recently detoxified alcoholics. Br,J Psychiatry 1994,16S: 515-23.
16 Pike VW. Positron-emitting radioligands for studies in vivo: probes for human psychopharmacology. J Psychopharmacol 1993; 7: 139-58.
17 London ED, Cascella NG, Wong DF, et al. Cocaine-induced redaction of glucose utilization in human brain. Arch Gen Psychiatry 1990; 47s 567-74.
18 Volpicelli JR Alterman AI, Haysshida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen fohiatry 1993; 49: 876-80.
19 Knob GR Drugs of abuse: anatomy, pharmacology and function of reward pathways. TIPS 1992;13:177-84.
20 Acquas E, Meloai M, Di Chiara G. Blockade of 8-opioid receptors in the nucleus accumbens prevent& ethanol-induced stimulation of dopamine release. EurJ Phormcd 1993; 230: 239.41.
21 Spanagel R Herz A, Shippenberg TS. The effects of *old peptide on dopamine release in the nutieus aeeumbens: an in vivo microdislysis study. I Narrod em 1990; SS: 1743-40.
22 Barterutein PA, Duncan JS, Prevett MC, a al. Investigation of opioid system in absence seizures with positron emission tomography, Nord Neuronal Poddany 1993; S6s 1295-302.
23 Gold MS; Redmond DE, Kkber HD Clomidine in opiate wkbdrawaL Lamxt 1978; ii: 599-602.
24 Brunnint J, Mumford Jl', Keartey PP Lofexidine in alcohol withdrawal statea. Akohrrl Akoholimr 1986; 21: 167-70.
25 Glue P, Nutt.Dj. Clanidine in alcohol withdrawal: a pilot study of differentisil symptom responses following is , cloaidme. Akoboi Akokofsae 1987,22s 161-66.
26 Bong S, Kvande H, Sedvall G. Central noepinephrine metabolism during alcohol inaoatkatim in addicts and healthy volunteers. Science 1981; 213:1135-37.
27 McDougle Cj, Black JE, Maliaon RT, a al. Noradrenerpe dyueguiadon during discomirsuatioa ofcoaine use in addicts. Arch Gnu Apckj 994; Si: 713-I9.
28 Cools AR. rential role of inineralocortiooid and gluoncortiooid receptors in the gargle of aced seadtbadw of mesolimlde, a t odrtaaergiareceptors in the ventral striatum: Naaoscimor 1991; 43s 41926.
29 Pike V W, Home SP, Aigbirhio F, Tirton DR Nutt Dj. [I 1-CJRX821002 as potential PET ts4ioligand for central adanoaptoml Label Cowporaed Radkpherm 1993; 321495-97.
30 Virklnmen M, Rawlings R 7bkola R a al. CSF biochamistrka, glucose metabolism, and diurnal activity rhythms in alcoholic, violent offenders, fat setters, and healthy volunteers. Arch Gen Porldatry 1994; Sir 20-27.
31 Beniselfat G Murphy Dl, Hill JL, George DT, Nam DJ, Lbmoila M. Ethmol-like properties of the serotatin partial agonist m-chlorphe::ylpiperazine in chronic akxbolic patienm Atch Gnu Ptyddarry 1991; 48: 383.
32 Uyers RD, Lankford M, Block A. Selective reduction by the 5-HT antagonist ampeaide ofak:ohol prefsaaee induced in cars; by systemic cyanamide. Pkaraiaad Biocbem Behw 1992; 43: 661-67.
33 Sellers EM, Huns GA, Sobell MB. 5-HT and alcohol abuse. 77PS 1992;13:69-75.
34 Kronder HR Budaon JA. Del Boa FK, a at. Btnpitona treament of anttiw elcoholim Ask Gas AOxhdarry 1994; 51:720-31.
35 Pike V W, McCaeon JA, Lammasmu AA, a al. First delineation of 5HT, receptors in human brain with PET and [°CJWAY 100635. P;rrr,J Pharmaeol 1995, 283: RI-R3.
36 Cowley DS, Roy-Byrne PP. Godon C, et al. Response to diazepam in sans of alcoholics. Akohd gin Fxp Re. 1"2,16:1057-63.
37 Nutt Dh Peters TJ• Aitohoh the drug. Br Med Bug 1994; Sos 5-17.
38 Trulsllo KA, Akil H. Inhibition of morphine tolerance and dependence by due NMDA receptor antagonist MK-801. Science 1991; 251: 85-87.
39 Person A, Paoli S, Swahn CG, HaBdin C, Sedvall G. Cerebral uptake of [l IIC-Ro 15-1788 and Its add metabolite 111JC-Ro 15-3890; PET study in healthy volunteers. Rmn Apchopharit 1989; 4: 215-20.
40 Woods SW, Sabyt JP, Goddard AW, et al. Dynamic SPECr imaging after iniecdost of the benzodiaxepine receptor ligand (123)1 in healthy human subleea. AOvWany Ra Nmviwa~ 1992; 4St 67-77.
41 Ma8xia AL, Couplaod NJ, Nun D7 Sassodianpioe receptor function in anxiety disorders: In: Brio G, Sauna E, San M, Costa E, ca. GABA-A receptors and anxiety: from neurobiology to treatment New York Raven Press, 1955:115-33.
42 Pact JA. Psychotropk drug tolerance and dependence: common underlying mechanisms? In: Pitt J. ed. The biological bases of drug tolerance and dependence. London: Academic Press, 1991:1--28.
43 Cosn11 B, Domeney AM, Hughes J. Kelly ME, NayW RJ. Woodnrf GN. AmdollKk e Is - s of CCK-B s ntagonistLNaavyrpdda 1991;19 (suppl): 65-73.
44 Abood ME, Martin BR Neurobiology of mwyuux abuse. 77BS 1992; 13t 201-06.
45 Devane WA, Hants 1, Brewer A, a al. Isolation and structure of a brain constituent that birds to the annrllibtoid receptor. Sdsna 1992; 158:1946-49.
46 Rinaldi-Carmona M, Barth F, Hauhne M, a al. SR141716A, a potent and selective antagonist of the brain annabinoid receptor. FEES Lea 1994; 3S0t 240-44.
47 Little HJ: The role of neuronal calcium channels in dependence of ethanol and other sedativez1hypnotim Pharmae 77u r 1991; SO: 347-65.












